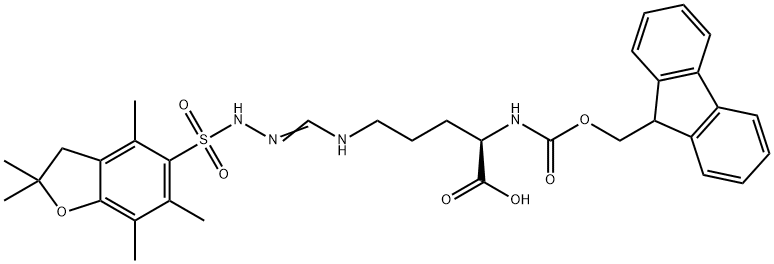
Synthesis and Application of Fmoc-D-Arg(Pbf) -OH
General description
Fmoc-D-Arg(Pbf)-OH is an amino acid derivative, which can be used as a pharmaceutical intermediate.
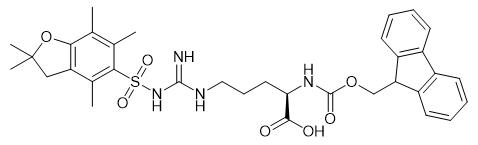
Fig. 1 The structure of Fmoc-D-Arg(Pbf)-OH.
Preparation
(1) Esterification
a1. Add 100 L of anhydrous methanol into a pre-dried 300 L reaction kettle, cool to -5 ~ -10°C with ice-cold brine, and drop 13 L of dichlorosulfoxide.
bl. Add 21.5 kg of L-type Arg. HCl, turn off the ice-cold brine, and naturally raise the temperature to room temperature for 24 hours.
cl. The reaction was heated to 35°C, followed by TLC point plate, and the reaction was finished in about 48 hours.
dl. Concentration: At the end of the reaction, the Arg · OMe · 2HCl intermediate of the oil is concentrated under reduced pressure.
(2) Introduce Boc group
a2. 150 L water was added into the 300 L reaction kettle, and then 25.2 kg sodium bicarbonate was added, and the mixture was stirred; Arg · OMe · 2HCl oil was added gradually; 30 L tetrahydrofuran was added; Add (Boc)2O, 26.16 kg in batches, stir, and react at room temperature; TLC point plate was used to track the reaction, and the treatment was started when the Arg·OMe·2HCl reaction was finished.
b2. Acidification: After the reaction, adjust pH 3-4 and extract with petroleum ether/ethyl acetate (50L:25L).
c2. Add salt to saturate, adjust pH 6-7, extract the product with ethyl acetate, and wash the organic phase with saturated salt water.
d2. Drying: Add 70 kg of anhydrous sodium sulfate to the organic phase (in the ethyl acetate solution with the product) and dry for 8 hours.
e2. Purification and concentration: sodium sulfate solid is filtered out and ethyl acetate phase is distilled under reduced pressure. The BocArgOMe. HCl oil is obtained after concentration.
(3) Introduce Pbf
a3 · Add Boc-ArgOMe · HCl, PBF-Cl 31.9 kg, acetone 200 L, potassium carbonate 41.7 kg, stir and add a small amount of water into the 300 L reaction kettle; The temperature was maintained at 40-45 °C, and the reaction was monitored with TLC until the BocArgOet. HCl reaction was complete.
b3. Extract and filter to remove solid insoluble matter, distill acetone under reduced pressure, and obtain Boc-Arg (Pbf) OMe oil after concentration for use.
(4) Remove Boc
a4. In a dry 300 L reaction kettle, add 120 L of 3N HC1/ ethyl acetate solution, add BoC-ARG (Pbf) OMe.HCl oil under stirring, maintain the temperature at 10-15°C. Stir at room temperature.
b4. After deBoc is completed, add water and wash the product to the aqueous phase, and add sodium carbonate to adjust the aqueous phase pH7.
(5) Saponification
a5. Add the above water to 100 L of 95% ethanol, stir, and drop 10N NaOH aqueous solution to adjust pH 11-12 for saponification.
b5. After the saponification reaction, the pH value of the reaction solution was adjusted to 7 with 6N HCl, and then cooled to -10-0°C for freezing crystallization; After centrifugation, the solid was stirred and washed once with ethyl acetate, and the solid was collected by centrifugation and drying. Recrystallization yields H-Arg (Pbf) OH solid.
(6) Fmoc-Arg (Pbf)-OH synthesis
a6 · Add H-Arg (Pbf)-OH, 120 L water, THF in the reaction kettle, adjust pH=8.5 with Na2CO3.
b6. Gradually add Fmoc-Osu (methoxycarbonylsuccinimide), control the temperature 15-20°C, pH=8-9 to complete the reaction of Arg (Pbf), and try to avoid excessive Fmoc-Osu. The TLC point plate was used to track the reaction. The reaction time was calculated from the end of Fmoc-Osu, and the reaction time was 6 hours.
c6. Purification: Extraction with petroleum ether/ethyl acetate (2:1); The aqueous phase was acidified with HCl to pH = 3 and stirred for 2 hours. Acidification temperature -0-10°C; Adding ethyl acetate to extract the product; Wash with saturated salt until PH reaches 6; Add anhydrous sodium sulfate to dry, 8 hours; The solid sodium sulfate is removed by vacuum filtration, the filtrate is concentrated, the solid is obtained by reducing pressure concentration, and the product is obtained by vacuum drying. The purity of the product is 99.5%, the largest single impurity is 0.11%, and the D-type isomer is 0.17% [1].
Application
For the synthesis of etelcalcetide
Secondary hyperparathyroidism (SHPT) refers to the condition of chronic renal insufficiency, intestinal malabsorption syndrome, Fanconi syndrome and renal tubular acidosis, vitamin D deficiency or resistance, pregnancy and lactation, etc. Parathyroid glands secrete excessive parathyroid hormone (PTH) in response to long-term stimulation of hypocalcemia, hypomagnesemia, or hyperphosphosis, which is a chronic compensatory clinical manifestation to increase serum calcium, magnesium, and decrease serum phosphorus. Long-term parathyroid hyperplasia eventually leads to the formation of functional autonomous adenoma. etelcalcetide is prepared by KaiPharmaceutica1s, Inc. and developed a novel meticiagent (calcimimeticagent), which can inhibit the secretion of parathyroid hormone. Veracartide can bind to and activate calcium sensitive receptors on parathyroid glands and reduce parathyroid hormone levels [2].
References
[1] Fu R, Zheng Z, Peng Z, et al. Synthesis method of Fmoc-arg(Pbf)-OH[P]. Faming Zhuanli Shenqing, 106928171, 2017.
[2] Gu H, Jin J, Liu T, et al. Preparing etelcalcetide, comprises e.g. subjecting fmoc-protected amino resin, fully protected amino acid and Fmoc-D-Arg(Pbf)-D-Arg(Pbf)-D-Arg(Pbf)-OH tripeptide fragment to condensation system, and performing resin cleavage reaction[P]. Faming Zhuanli Shenqing, 109734778, 2019.
See also
Lastest Price from Fmoc-D-Arg(Pbf)-OH manufacturers
US $0.00-0.00/kg2025-04-21
- CAS:
- 187618-60-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1T
US $1.10/g2025-04-17
- CAS:
- 187618-60-6
- Min. Order:
- 1g
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min