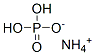Unique properties of ammonium dihydrogen phosphat
Introduction
Ammonium dihydrogen phosphate, a chemical agent, also known as monoammonium phosphate, is a white crystal with the chemical formula of NH4H2PO4[1]. When heated, it will decompose into ammonium metaphosphate (nh4po3), which can be made by the reaction of ammonia and phosphoric acid. It is a white crystalline powder. Stable in air. Slightly soluble in ethanol, insoluble in acetone. The aqueous solution is acidic. The solubility in water at room temperature (20 ℃) is 37.4g. The relative density is 1.80. Melting point 180 ℃. The refractive index is 1.525. White crystalline powder. Stable in air. Slightly soluble in ethanol, insoluble in acetone. The aqueous solution is acidic. The solubility in water at room temperature (20 ℃) is 37.4g. The relative density is 1.80. Melting point 180 ℃. The refractive index is 1.525. When ammonium dihydrogen phosphate is transported in woven bags, the woven bags are lined with plastic film and sewn and sealed. Pay attention to sunscreen, moisture-proof, waterproof and broken bags to avoid loss.

Picture 1 Ammonium dihydrogen phosphate powders
Properties
It was first shown by Busch that potassium dihydrogen phosphate KH2P04 and several isomorphous salts, potassium dihydrogen arsenate KH2ASO4, ammonium dihydrogen phosphate NH4H2PO4, and ammonium dihydrogen arsenate NH4H2ASO4 exhibited phase changes at low temperatures[2]. These crystals all exhibit phase changes at temperatures ranging from 91° absolute to 220° absolute temperature. It was established for potassium dihydrogen phosphate (which will be designated by the letters KDP) and potassium dihydrogen arsenate (KDA) by measuring the dielectric constants and the associated charge potential loops that these phase changes were of the ferroelectric type. Similar measurements of ammonium dihydrogen phosphate (ADP) and ammonium dihydrogen arsenate (ADA) failed to show ferroelectric properties on account of the sudden fracture of these crystals at temperatures above the ferro[1]electric Curie temperatures.
Tunable parametric amplification of quantum noise in ammonium dihydrogen phosphate
This Letter reports the quantitative experimental study of tunable parametric amplification of quantum noise in ammonium dihydrogen phosphate (ADP). Because of phase-matching conditions the parametric noise output is limited to narrow spectral regions which could be tuned from 4400 to 16 000 A by simply rotating the ADP crystal around the pump-beam direction[3]. A complete study of the physical parameters which determine this spontaneous parametric interaction was made and the results compared with theory. The results are also of practical interest (a) because the observed small levels of output represent the noise level of parametric light amplifiers and (b) because the measured gain allows predictions about the construction of tunable coherent light oscillators in the visible. The extension of tunable parametric output through the visible and the direct measurement of various physical parameters of single-pas[1]sage amplification without the use of external mirrors differentiates the reported measurements from previous observations of tunable parametric oscillators. Powerful coherent output in the infrared and far red using an op tical cavity and a pump at 5300 A has been observed by Giordmaine and Miller1 and Miller and Nordland in LiNbOs and by Akhmanov et al. in potassium dihydrogen phosphate.
The Elastic, Piezoelectric, and Dielectric Constants of Potassium Dihydrogen Phosphate and Ammonium Dihydrogen Phosphate
Measurements have been made of all the elastic, piezoelectric, and dielectric constants of KDP and ADP crystals through temperature ranges down to the Curie temperatures[4]. The piezoelectric properties agree well with Mueller's phenomenological theory of piezoelectricity provided the fundamental piezoelectric constant is taken as the ratio of the piezoelectric stress to that part of the polarization due to the hydrogen bonds. It is found that the dielectric properties of KDP agree well with the theory presented by Slater based on the interaction of the hydrogen bonds with the PO4 ions. ADP undergoes a transition at -125°C which results in fracturing the crystal. This transition cannot be connected with the H2PO4 hydrogen bond system which controls the dielectric and piezoelectric properties, for these lie on smooth curves that do not change slope as the transition temperature is approached. It is suggested that two separate and independent hydrogen bond systems are involved in ADP. The transition temperature and specific heat anomaly appear to be connected with hydrogen bonds between the nitrogens and the oxygens of the PO4 ions, while the dielectric and piezoelectric properties are controlled by the H2PO4 hydrogen bonds.
Reference
1 Davey, R. J., and J. W. Mullin. "Growth of the {100} faces of ammonium dihydrogen phosphate crystals in the presence of ionic species." Journal of Crystal Growth 26.1 (1974): 45-51.
2 Zernike F. Refractive indices of ammonium dihydrogen phosphate and potassium dihydrogen phosphate between 2000 Å and 1.5 μ[J]. JOSA, 1964, 54(10): 1215-1220.
3 Magde D, Mahr H. Study in ammonium dihydrogen phosphate of spontaneous parametric interaction tunable from 4400 to 16 000 Å[J]. Physical Review Letters, 1967, 18(21): 905.
4 Mason W P. The elastic, piezoelectric, and dielectric constants of potassium dihydrogen phosphate and ammonium dihydrogen phosphate[J]. Physical Review, 1946, 69(5-6): 173.
You may like
Related articles And Qustion
See also
Lastest Price from Ammonium dihydrogen phosphate manufacturers

US $550.00-480.00/Ton2025-07-01
- CAS:
- 7722-76-1
- Min. Order:
- 27Ton
- Purity:
- 99.9%
- Supply Ability:
- 5000tons

US $0.00-0.00/Ton2025-07-01
- CAS:
- 7722-76-1
- Min. Order:
- 27Ton
- Purity:
- 99%
- Supply Ability:
- 5000tons