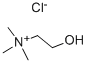
Properties and structure of choline chloride
Introduction
Choline chloride is an organic substance with the chemical formula C5H14CINO. It is white, hygroscopic crystal, tasteless and fishy. Melting point: 305 ℃. 10% aqueous solution pH 5-6, unstable in alkaline solution. This product is easily soluble in water and ethanol, but insoluble in ether, petroleum ether, benzene and carbon disulfide. Low toxicity, LD50 (rat, oral) 3400 mg / kg. For the treatment of fatty liver and liver cirrhosis. It is also used as a feed additive for livestock, which can stimulate the ovaries to produce more eggs, litter, and increase the weight of livestock and fish[1].

Picture 1 Choline chloride powders
Structure
At room temperature choline chloride is remarkably sensitive to decomposition into trimethylamine and acetaldehyde by ionizing radiations because of a chain reaction which occurs extensively only in the solid state[2]. We have determined the structure of this crystal and have made preliminary studies of choline bromide and choline iodide, which are more stable, to aid in the interpretation of this phenomenon. Collin discovered that at elevated temperature choline chloride changes to a disordered cubic phase, which is also more stable than the room-temperature phase with respect to radiation damage.
Choline iodide is monoclinic, probable space group P21. Crystals of choline chloride after recrystallization from dimethyl formamide were colorless, transparent needles with the long dimension in the c direction. Their hygroscopic nature made it necessary to mount them in glass capillaries. After about 40 hr. of exposure to nickel-filtered copper X-rays under normal diffraction conditions they became opaque and slightly yellow and yielded very diffuse diffraction spots. This in[1]stability limited the available diffraction data to reflections with sin 2 0 < 0.60. The atomic positions are derived from intensity data from a set of multiple[1]film Weissenberg photographs for /=0 to 4 with rotation about the c axis. The calculations are based on a total of 357 independent reflections including 72 too weak to be observed. The systematic absences correspond to space group P212~2~, and a satisfactory structure was found with that symmetry.
Novel solvent properties of choline chloride/urea mixtures
Eutectic mixtures of salts have been utilised for a long time to decrease the temperature for molten salt applications[3]. In the extreme, ambient temperature molten salts have been formed by mixing quaternary ammonium salts with metal salts. This type of ionic liquid can be viewed as a deep eutectic resulting from the formation of complex anions thus decreasing the lattice energy and decreasing the freezing point of the system. Work in this area focussed on chloroaluminate salts of imidazolium and pyridinium chloride. A variety of different anions are formed in solution the ratios of which vary with the changing aluminium chloride composition. These ideas have recently been extended to other chlorometallate salts including ZnCl2, SnCl2 and FeCl
Halide salts can also form complexes with hydrogen bond donors and previous work has shown that mixtures of urea with alkali metal halides form eutectics with melting points of <150 °C. While a few reports also exist for adducts of urea with other metal salts their use as solvents has been limited to high temperature applications.10 In the current work we show that mixtures of substituted quaternary ammonium salts such as hydroxyethyltrimethylammonium (choline) chloride with urea produce eutectics that are liquid at ambient temperature and have unusual solvent properties. The freezing point of mixtures of choline chloride and urea. A eutectic occurs at a urea to choline chloride ratio of 2. The freezing point of the eutectic mixture is 12 °C, which is considerably lower than that of either of the constituents (mp choline chloride = 302 °C and urea = 133 °C) and allows the mixture to be used as an ambient temperature solvent. This significant depression of the freezing point must arise from an interaction between urea molecules and the chloride ion. This is consistent with the crystallographic data for the solid adduct [(CH3)3N+CH2CH2OH]2C2O42−·2(NH2)2CS which shows extensive hydrogen bonding between the thiourea molecule and the oxalate anion. Previous work has also shown that the stable adduct Pr4N+Cl·2(NH2)2CO can be isolated although this has a melting point of 160 °C. The existence of hydrogen bonding in these eutectic mixtures can be observed using NMR spectroscopy. HOESY spectra of HOCH2CH2N+(CH3)3F−·2(NH2)2CO show intense cross-correlation between the fluoride ion and the NH2 protons on the urea molecule. FAB-MS which was run neat on a choline chloride∶urea mixture without a matrix showed the presence of Cl− with two ureas (M− = 155) and Cl− with one urea (M− = 95).
Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids
Deep Eutectic Solvents (DES) can be formed between a variety of quaternary ammonium salts and carboxylic acids. The physical properties are significantly affected by the structure of the carboxylic acid but the phase behavior of the mixtures can be simply modeled by taking account of the mole fraction of carboxylic acid in the mixture. The physical properties such as viscosity, conductivity, and surface tension of these DES are similar to ambient temperature ionic liquids and insight into the cause of these properties is gained using hole-theory. It is shown that the conductivity and viscosity of these liquids is controlled by ion mobility and the availability of voids of suitable dimensions, and this is consistent with the fluidity of other ionic liquids and molten salts. The DES are also shown to be good solvents for metal oxides, which could have potential application for metal extraction[4].
Reference
1 Abbott, Andrew P., et al. "Novel solvent properties of choline chloride/urea mixtures." Chemical communications 1 (2003): 70-71.
2 Senko M E, Templeton D H. Unit cells of choline halides and structure of choline chloride[J]. Acta Crystallographica, 1960, 13(4): 281-285.
3 Abbott A P, Capper G, Davies D L, et al. Novel solvent properties of choline chloride/urea mixtures[J]. Chemical communications, 2003 (1): 70-71.
4 Abbott A P, Boothby D, Capper G, et al. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids[J]. Journal of the American Chemical Society, 2004, 126(29): 9142-9147.
Related articles And Qustion
See also
Lastest Price from Choline chloride manufacturers

US $0.00-0.00/kg2025-09-30
- CAS:
- 67-48-1
- Min. Order:
- 1kg
- Purity:
- 98.0%-100.5%;FCCV
- Supply Ability:
- 10 TONS

US $1200.00-1100.00/ton2025-09-25
- CAS:
- 67-48-1
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M