Biological functions and Synthesis of Hydrogen sulfide
Hydrogen sulfide is important in the regulation of many cellular processes and is strongly implicated in oxygen sensing.
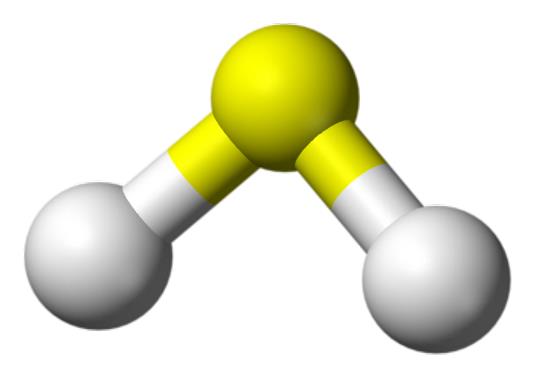
Structure
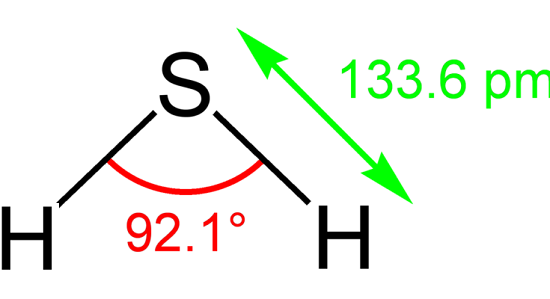
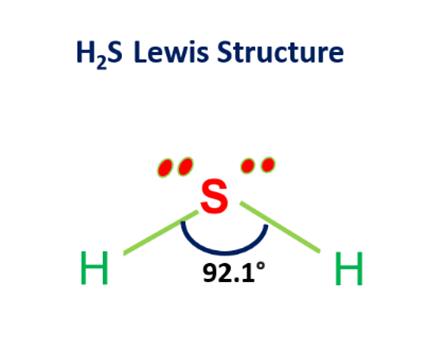
H2S is a 34.08Da molecule that has a molecular charge of zero and a tetrahedral electron pair geometry with two pairs of bonding electrons and two lone pairs. The molecule is slightly polar and a strong reductant. H2S is both hydrophilic and lipophilic. In solution,H2S is a weak acid.In intracellular fluid, nearly equal amounts of H2S and HS- are present, but in the extracellular fluid there is approximately a 1:4 ratio of H2S to HS-at 37°C and pH7.4. Changes in pH between the cytosol and mitochondria will affect the H2S/HS- ratio. Generally, the term H2S is used to reflect the sum of the H2S/HS-/S2- species.
Synthesis and release
H2S synthesis primarily occurs via enzyme-catalyzed reactions. Themajority ofH2S is synthesized using cysteine as a precursor. The enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), synthesize H2S directly from homocysteine and cysteine. CBS and CSE can translocate to the mitochondria during cellular stress and generate H2S from cysteine in the mitochondrial matrix.
Cysteine can also be converted to 3-mercaptopyruvate (3-MP) by cysteine aminotransferase (CAT) and H2S is generated by the enzyme 3-mercaptopyruvate sulfur transferase (3-MST) in the presence of a reductant.H2S is the only gasotransmitter that is enzymatically inactivated with the catabolismoccurring in the mitochondria. H2S binds to sulfur quinone oxidoreductase forming a persulfide and the transfer of two electrons into the electron transport chain that ultimately leads toATP production. Persulfide is further metabolized through a number of complex pathways in the mitochondria. Polysulfides (H2Sn) synthesized in the 3-MST pathway are now recognized as important signaling molecules in addition to H2S.
Biological functions
H2S regulates a vast array of functions at the cellular and systems levels. At the cellular level, H2S controls cell survival, differentiation, hypertrophy, and apoptosis, and is also cytoprotective by reducing oxidative stress.18 In the cardiovascular system, H2S is a specific activator of KATP channels in VSM cells and inhibits cGMP phosphodiesterase, which enhances vasodilation mediated by nitric oxide. KCa, Cl-, and Ca2+ channels are also cellular targets for H2S. H2S is also important in regulating respiratory, renal, hepatic, endocrine, gastrointestinal, immune, and reproductive systems. It is proposed that H2S is an “oxygen sensor” in key tissues in vertebrates.
Clinical implications
The dysfunction of H2S production and signaling is implicated in a range of diseases, including respiratory and cardiovascular diseases, atherosclerosis, cancer, diabetes, erectile dysfunction, hypertension, neurodegenerative diseases, and oxidative stress associated with ischemia-reperfusion and injury.