Application of Cyclohexylamine
General description
Cyclohexylamine appears as a clear colorless to yellow liquid with an odor of ammonia. Flash point 90°F. Irritates the eyes and respiratory system. Skin contact may cause burns. Less dense than water. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion. Cyclohexylamine is a primary aliphatic amine consisting of cyclohexane carrying an amino substituent. It has a role as a human xenobiotic metabolite and a mouse metabolite. It is a conjugate base of a cyclohexylammonium. Cyclohexylamine is a natural product found in Zanthoxylum with data available. Cyclohexylamine, an organic compound with the chemical formula c6h13n, is a colorless to yellow liquid, soluble in water and miscible in most organic solvents. It is mainly used as a solvent and can also be used to prepare desulfurizer, rubber antioxidant, vulcanization accelerator, plastic and textile chemical additives, boiler feed water treatment agent, metal corrosion inhibitor, emulsifier, preservative, antistatic agent, latex coagulant, petroleum additive, bactericide Insecticide and dye intermediates.
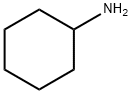
Figure 1 the chemical structure of Cyclohexylamine
Application and Pharmacology
Cyclohexylamine is a flammable and toxic organic pollutant. In the process of production and use, the released cyclohexylamine waste gas is very harmful to the environment and human body. Microbial degradation of organic pollutants has the advantages of low cost and high efficiency. Therefore, it is very important to find a microorganism that can degrade cyclohexylamine and study its degradation mechanism to reduce the pollution of cyclohexylamine in the environment. At present, there are mainly two pure cultures grown with cyclohexylamine as carbon source and nitrogen source, one of which is from iwakih Brevibacteriumoxydansih-35a and pseudomonasplecoglossicidanyz12 were isolated by zhouningyi research group of Wuhan Institute of Virology, Chinese Academy of Sciences. The whole genome information of the strain was obtained by sequencing the whole genome of nyz12[1]. Cyclohexylamine (A6c = 1-aminocyclohexane carboxylic acid) is a synthetic antimicrobial peptide (AMP) that exhibits in vitro inhibitory activity against drug resistant strains of Staphylococcus aureus, Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterobacter aerogenes, and Enterococcus faecium at concentrations ranging from 10.9 to 43 lM. Spectroscopic investigations were conducted to determine how this AMP interacts with simple membrane model systems in order to provide insight into possible mechanisms of action. CD and 2D-1H NMR experiments indicated this AMP on binding to SDS and DPC micelles adopts conformations with varying percentages of helical and random coil conformers. CD investigations in the presence of three phospholipid SUVs consisting of POPC, 4:1 POPC/POPG, and 60% POPE/21%POPG/19%POPC revealed: (1) The interactions occurring with POPC SUVs have minimal effect on the conformational diversity of the AMP yielding conformations similar to those observed in buffer. (2) The interactions with 4:1 POPC/POPG, and 60% POPE/21%POPG/19%POPC SUVs exhibited a greater influence on the percentage of different conformers contributing to the CD spectra. (3) The presence of a high of percentage of helical conformers was not observed in the presence of SUVs as was the case with micelles. This data indicates that the diversity of surface bound conformations adopted by this AMP are very different from the diversity of conformations adopted by this AMP on insertion into the lipid bilayer. CD spectra of this AMP in the presence of SUVs consisting of LPS isolated from P. aeruginosa, K. pneumoniae and Escherichia coli exhibited characteristics associated with various helical conformations[2].
Synthesis
An excess of Mg(AlH4)2 tetrahydrofuranate is a powerful reducing agent for Me2CO (to give 100% Me2CHOH), Ph2CO (100% Ph2CHOH), cyclohexanone oxime (100% cyclohexylamine), PhNO2 (100% PhN:NPh), BzNH2 (100% PhCH2NH2), BzOH (100% Ph-CH2OH), MeOBz (100% PhCH2OH), crotonaldehyde (100% crotyl alc.), Bu3SnCl (100% Bu3SnH), PhCH:CHCO2H (∼60% PhCH:CHCH2OH and ∼30% PhCH2CH2CH2OH), and PhCH:CHCO2Me (∼75% PhCH2CH2CH2OH). PhBr did not react[3].
Safety
First aid measures editor broadcast skin contact: take off contaminated clothes immediately and wash with a large amount of flowing water for at least 15 minutes. Seek medical attention. Eye contact: lift the eyelids immediately and wash thoroughly with a large amount of flowing water or normal saline for at least 15 minutes. Seek medical attention. Inhalation: quickly leave the site to a place with fresh air. Keep respiratory tract unobstructed. If breathing is difficult, give oxygen. If breathing stops, give artificial respiration immediately. Seek medical attention. Ingestion: rinse with water and drink milk or egg white. Seek medical attention.
Reference
1.Cyclohexylamine degrading bacterium nyz12 Construction and characterization of knockout mutants_ Maolingqi.
2.Abercrombie J. J., Leung K. P. & Chai H. et al., "Spectral and biological evaluation of a synthetic antimicrobial peptide derived from 1-aminocyclohexane carboxylic acid," Bioorganic & Medicinal Chemistry, Vol.23, No.6(2015), pp.1341-1347.
3.Huanglongjun, baojiahao, guoluyao, etc.: ru/zro_2 catalytic reduction amination of cyclohexanone to prepare cyclohexylamine, chemical reaction engineering and technology, 2019, issue 03, page 274-281.
You may like
Related articles And Qustion
See also
Lastest Price from Cyclohexylamine manufacturers

US $1.00/KG2025-04-21
- CAS:
- 108-91-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 108-91-8
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons