Tungsten trioxide - Uses, Toxicity, Preparation etc.
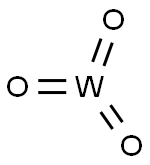
Tungsten(VI) oxide, also known as tungsten trioxide or tungstic anhydride, WO3, is a chemical compound containing oxygen and the transition metal tungsten.
Tungsten trioxide is a bright canary-yellow coloured powder which becomes dark-orange on heating, but regains its bright yellow colour on cooling. A very slight admixture of sodium salt imparts to the oxide a greenish tint which no amount of oxidation can remove (Roscoe). It also becomes greenish on exposure to light.

Tungsten trioxide has been obtained in the crystalline state by Debray, by igniting a mixture of tungstate and carbonate of sodium in a current of hydrochloric acid, when the trioxide is obtained in olive-green rectangular prisms which sublime at a white heat. The crystalline trioxide has also been prepared by heating hydrated tungstic acid with borax in a porcelain furnace (Nordenskjold).
When heated in a current of hydrogen to 250° the trioxide is converted into the blue oxidc, 2WO3+WO2 and if the heat be raised to dull redness the brown oxide, WO2 is formed, whilst at a higher temperature the metal is obtained. Acted upon by reducing agents such as zinc and hydrochloric acid, stannous chloride, or organic matter, tungsten trioxide is trans-formed successively into the blue and brown oxides.
Uses
Tungsten trioxide is a chemical compound consisting of oxygen and the transition metal tungsten. It occurs naturally but rarely in the form of hydrates in some kinds of minerals. It has various applications. In industry, it can be used for making x-ray screen phosphors, fireproofing fabrics and gas sensors as well as a pigment in ceramics and paints. It can also be used for the preparation of electrochromic windows or smart windows. In addition, it can be used in semiconductors. It, together with titanium dioxide, has the potential to become an efficient photo-catalyst under visible light irradiation. Recent study has shown that mesoporous tungsten trioxide polyaniline nanocomposite can be used as an anode material for high-performance lithium-ion batteries.
Tungsten trioxide is a chemical compound consisting of oxygen and the transition metal tungsten. It occurs naturally but rarely in the form of hydrates in some kinds of minerals. It has various applications. In industry, it can be used for making x-ray screen phosphors, fireproofing fabrics and gas sensors as well as a pigment in ceramics and paints. It can also be used for the preparation of electrochromic windows or smart windows. In addition, it can be used in semiconductors. It, together with titanium dioxide, has the potential to become an efficient photo-catalyst under visible light irradiation. Recent study has shown that mesoporous tungsten trioxide polyaniline nanocomposite can be used as an anode material for high-performance lithium-ion batteries. Tungsten trioxide is a thermally stable and water insoluble tungsten compound. Tungsten trioxide andTungsten blue oxides are both used in the production of tungsten metal powders. The greatmajority of metallic tungsten is used to make cemented carbide parts, and theremainder is used in manufacturing components for lighting, electrical, electronic,heating, and welding applications; and in tool steels, alloys, catalysts, pigments, andhigh-temperature lubricants.
Tungsten trioxide is used for many purposes in everyday life. It is frequently used in industry to manufacture tungstates for x-ray screen phosphors, for fireproofing fabrics and in gas sensors.Due to its rich yellow color, WO3 is also used as a pigment in ceramics and paints. In recent years, tungsten trioxide has been employed in the production of electrochromic windows, or smart windows. These windows are electrically switchable glass that change light transmission properties with an applied voltage. This allows the user to tint their windows, changing the amount of heat or light passing through.
Recently, some research groups have demonstrated that non-metal surface such as transition metal oxides (WO3, TiO2, Cu2O, MoO3, and ZnO etc.) could serve as a potential candidate for surface-enhanced Raman spectroscopy substrates and their performance could be comparable or even higher than those of commonly used noble-metal elements. There are two basic mechanisms for this application. One is that the Raman signal enhancement was tuned by charge transfer between the dye molecules and the substrate WO3 materials. The other is to use the electrical tuning of the defect density in the WO3 materials by the oxide leakage current control in order to modulate the enhancement factor of the SERS effect.
Preparation
Tungsten trioxide can be prepared in several different ways. CaWO4, or scheelite, is allowed to react with HCl to produce tungstic acid, which decomposes to WO3 and water at high temperatures.
CaWO4 + 2 HCl → CaCl2 + H2WO4
H2WO4 → H2O + WO3
Another common way to synthesize WO3 is by calcination of ammonium paratungstate (APT) under oxidizing conditions:
(NH4)10[H2W12O42] · 4 H2O → 12 WO3 + 10 NH3 + 10 H2O
Additionally, yellow tungsten trioxide can be produced by roasting ammonium paratungstate at closely controlled temperatures to drive off combined water and ammonia.
You may like
Related articles And Qustion
Lastest Price from Tungsten trioxide manufacturers
US $0.00-0.00/KG2025-12-13
- CAS:
- 1314-35-8
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00/KG2025-04-15
- CAS:
- 1314-35-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg