An oxide of rare earth metal europium: Europium oxide
Introduction
Due to the high reactivity with oxygen, the most stable compounds of rare-earth (RE) elements are their oxides with a general formula R2O3, called sesquioxides, in which the RE ions (R) exist in the trivalent state. The unique physical and chemical properties of the RE sesquioxides, such as the dense Kondo effect, heavy fermionic behavior, and high dielectric constants, just to mention a few, make them attractive from both scientific and technological perspectives.
Europium oxide exists in three stoichiometric forms. At lower oxygen pressure, europium oxidizes first to the NaCl-type EuO and then to the spinel-type Eu3O4 before the stable sesquioxide is formed. The trivalent Eu3+ ions with the 4f6 electron configuration (7 F0) have a total angular momentum of J = 0 and L = S = 3.
Eu2O3
Europium oxide(Eu2O3) is an oxide of rare earth metal europium. Europium oxide also has other names, such as Europia, Europium trioxide, and Dieuropium trioxide. Europium oxide has a pinkish-white color. Europium oxide has two different structures: cubic and monoclinic. The cubic structure of europium oxide is almost the same as the magnesium oxide structure. Europium oxide has negligible solubility in water but readily dissolves in mineral acids. Europium oxide is a thermally stable material that has a melting point of 2350 ℃. Europium oxide’s multi-efficient properties, such as magnetic, optical, and luminescence, make this material very important. Europium oxide can absorb moisture and carbon dioxide in the atmosphere. Europium oxide has great potential as a photoactive material for the photocatalytic degradation of organic pollutants.
Structure
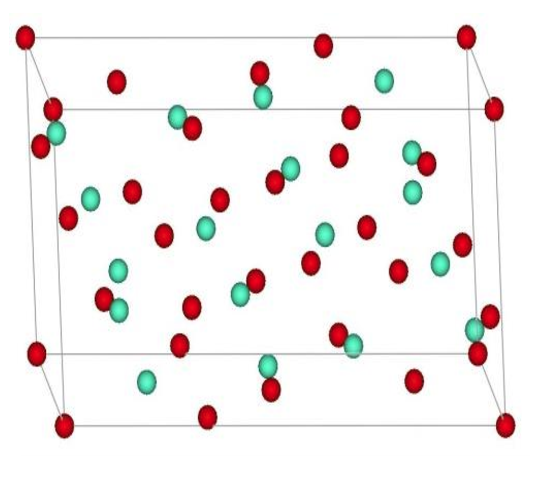
At ambient conditions, Eu2O3 crystallizes in a cubic (C) structure and experiences structural transformations to monoclinic (B), trigonal (A), hexagonal (H), and cubic (X) phases with increasing temperature. Under pressure, the structural transition from the cubic C-type to the trigonal A-type phase, which starts at 5.0 GPa and finishes at about 13.1 GPa, is observed. This transition leads to a volume collapse of 9% at 8.6 GPa. The trigonal phase remains stable up to the highest experimentally feasible pressure. After the release of the pressure, the trigonal phase transforms into the monoclinic phase. Also, a direct phase transition from the C-type structure to the B-type monoclinic structure was observed at about 8.0 GPa, and the B-type structure was retained after the pressure was released, indicating that the monoclinic phase is metastable at room temperature. A pressure-induced phase transition from the monoclinic to trigonal crystal structure was observed at about 4.7 GPa. Finally, at ambient conditions, Eu2O3 may exist in a stable cubic form and in a metastable B-type structure similar to the Sm2O3 and Gd2O3 compounds[1].
Reference
[1] Jan Łażewski*. “Lattice Dynamics and Structural Phase Transitions in Eu2O3.” Inorganic Chemistry 60 13 (2021): 9571–9579.
You may like
See also
Lastest Price from Europium Oxide manufacturers

US $30.00-27000.00/kg2025-06-09
- CAS:
- 1308-96-9
- Min. Order:
- 1000kg
- Purity:
- 99.5%
- Supply Ability:
- 20T per month

US $100.00/KG2025-04-21
- CAS:
- 1308-96-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt