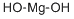Applications of Magnesium hydroxide
Magnesium hydroxide is a white to off-white crystalline powder with a specific gravity of 2.4 and a Mohs hardness of around 3.0. It loses 30.9% of its mass as water vapor on heating above 450°C. It being soluble in dilute acid and ammonium salt solution, but almost insoluble in water and alcohol. The solubility in water (18 ° C) was 0.0009g/100g.
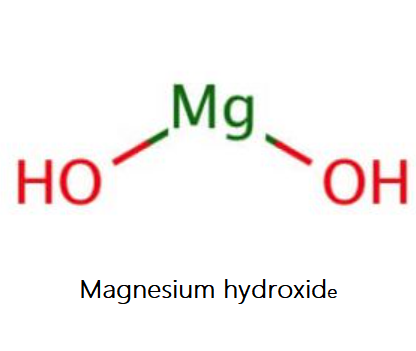
Magnesium Hydroxide is an inorganic compound with the formula Mg(OH)2. The solid mineral form of Magnesium Hydroxide is brucite, first described in 1824 and named after its discoverer, the American mineralist Archibald Bruce (1777-1818). Brucite is used as a flame retardant because it thermally decomposes in a similar way as aluminium hydroxide and mixtures of huntite and hydromagnesite, to release water. The superior properties of the synthetic form of Mg(OH)2, in terms of purity and particle size distribution,has opened up far more applications. Synthetic Magnesium Hydroxide is produced by Kyowa Chemical Industry since 1950 and by us, Kisuma Chemicals since 1998.

Uses
One important application of Mg(OH)2 is the use of the material in the pharmaceutical industry, for example as antacid to neutralize stomach acids and as laxative. Although the use of Mg(OH)2 for this purpose was already described in 1829 by Sir James Murray, who used a fluid magnesia preparation to treat the Lord Lieutenant of Ireland, the Marquis of Anglesey, of stomach pain, it was first sold under the brandname ''Philip's Milk of Magnesia'' by Charles Henry Philip's in 1872. Today, medicinal type Mg(OH)2 is sold in liquid form, chewable tablets or tablets.
The largest industrial application of Mg(OH)2 is flame retardants for articles such as roofing, isolation materials, plastic articles and coatings. The mechanism of flame retardancy is based on the endothermic decomposition of the material into MgO and H2O. This reaction adsorbs heat, which delays ignition of the associated substance. The water that is released dilutes combustible gases and inhibits oxygen from aiding the combustion. Other known applications of Mg(OH)2 include food additives, where the material is used as acidity regulator, and as precursor for other magnesium materials, most notably MgO.
The largest industrial application of Mg(OH)2 is flame retardants for articles such as roofing, isolation materials, plastic articles and coatings. The mechanism of flame retardancy is based on the endothermic decomposition of the material into MgO and H2O. This reaction adsorbs heat, which delays ignition of the associated substance. The water that is released dilutes combustible gases and inhibits oxygen from aiding the combustion. Other known applications of Mg(OH)2 include food additives, where the material is used as acidity regulator, and as precursor for other magnesium materials, most notably MgO.
This product has a variety of advantage including being smoke-free, non-toxic, non-corrosive, cheap and easy to get advantages. Moreover, the temperature of its decomposition for release of water is higher than aluminum hydroxide, being more suitable for the requirement of high-temperature processing. Magnesium hydroxide can be used as the flame retardants of polyethylene, polypropylene, PVC, EPDM, unsaturated polyester and other plastics and rubber. It can also be used as the flame retardant of paint.
History of milk of magnesia
On May 4, 1818, American inventor John Callen received a patent (No. X2952) for magnesium hydroxide. In 1829, Sir James Murray used a "condensed solution of fluid magnesia" preparation of his own design to treat the Lord Lieutenant of Ireland, the Marquis of Anglesey, of stomach pain. This was so successful (advertised in Australia and approved by the Royal College of Surgeons in 1838) that he was appointed resident physician to Anglesey and two subsequent Lords Lieutenant, and knighted. His fluid magnesia product was patented two years after his death in 1873.
The term milk of magnesia was first used by Charles Henry Phillips in 1872 for a suspension of magnesium hydroxide formulated at about 8%w/v. It was sold under the brand name Phillips' Milk of Magnesia for medicinal usage.
Although the name may at some point have been owned by GlaxoSmithKline, USPTO registrations show "Milk of Magnesia" and "Phillips' Milk of Magnesia" have both been assigned to Bayer since 1995. In the UK, the non-brand (generic) name of "Milk of Magnesia" and "Phillips' Milk of Magnesia" is "Cream of Magnesia" (Magnesium Hydroxide Mixture, BP).
As food additive
It is added directly to human food, and is affirmed as generally recognized as safe by the FDA. It is known as E number E528.
Magnesium hydroxide is marketed for medical use as chewable tablets, as capsules, powder, and as liquid suspensions, sometimes flavored. These products are sold as antacids to neutralize stomach acid and relieve indigestion and heartburn. It also is a laxative to alleviate constipation. As a laxative, the osmotic force of the magnesia acts to draw fluids from the body. High doses can lead to diarrhea, and can deplete the body's supply of potassium, sometimes leading to muscle cramps.
You may like
Related articles And Qustion
See also
Lastest Price from Magnesium hydroxide manufacturers

US $0.00-0.00/KG2025-12-12
- CAS:
- 1309-42-8
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $10.00/kg2025-04-21
- CAS:
- 1309-42-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt