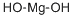Magnesium hydroxide: Preparation, applications and pharmacodynamics
General description
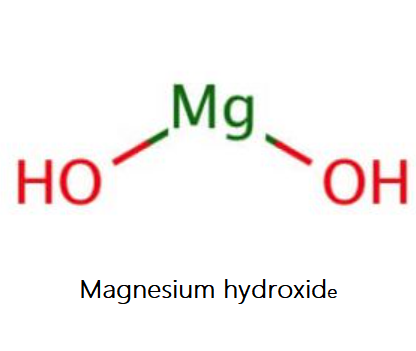
Magnesium hydroxide is the inorganic compound occurring in nature as the mineral brucite. Magnesium hydroxide is a common component of antacids, such as milk of magnesia. Its appearance is as follows:

Figure 1 Appearance of Magnesium hydroxide
Preparation
Combining a solution of many magnesium salts with alkaline water induces precipitation of solid Magnesium hydroxide. On a commercial scale, Magnesium hydroxide is produced by treating seawater with lime (Ca(OH)2). 600 m3 (158,503 US gallons) of seawater gives about one ton of Magnesium hydroxide. Ca(OH)2 is far more soluble than Magnesium hydroxide, so the latter precipitates as a solid.[1]
Applications
Most Magnesium hydroxide that is produced industrially, as well as the small amount that is mined, is converted to fused magnesia (MgO). Magnesia is valuable because it is both a poor electrical conductor and an excellent thermal conductor.[1] Magnesium hydroxide is used in suspension as either an antacid or a laxative, depending on concentration. As an antacid, magnesium hydroxide is dosed at approximately 0.5–1.5 g in adults and works by simple neutralization, where the hydroxide ions from the Magnesium hydroxide combine with acidic H+ ions produced in the form of hydrochloric acid by parietal cells in the stomach to produce water. Magnesium hydroxide is marketed for medical use as chewable tablets, as capsules, powder, and as liquid suspensions, sometimes flavored. These products are sold as antacids to neutralize stomach acid and relieve indigestion and heartburn. It also is a laxative to alleviate constipation. As a laxative, the osmotic force of the magnesia acts to draw fluids from the body. High doses can lead to diarrhea, and can deplete the body's supply of potassium, sometimes leading to muscle cramps.
Magnesium hydroxide is also a component of antiperspirant. Magnesium hydroxide is useful against canker sores (aphthous ulcer) when used topically. Magnesium hydroxide powder is used industrially to neutralize acidic wastewaters.[2] It is also a component of the Biorock method of building artificial reefs. Natural magnesium hydroxide (brucite) is used commercially as a fire retardant. Most industrially used magnesium hydroxide is produced synthetically.[3] Like aluminium hydroxide, solid magnesium hydroxide has smoke suppressing and flame retardant properties. This property is attributable to the endothermic decomposition it undergoes at 332 °C (630 °F).
Pharmacodynamics
As an antacid, magnesium hydroxide is dosed at approximately 0.5–1.5 g in adults and works by simple neutralization, where the hydroxide ions from the Mg(OH)2 combine with acidic H+ ions produced in the form of hydrochloric acid by parietal cells in the stomach to produce water.
As a laxative, magnesium hydroxide is dosed at 2–5 g, and works in a number of ways. First, Mg2+ is poorly absorbed from the intestinal tract, so it draws water from the surrounding tissue by osmosis. Not only does this increase in water content soften the feces, it also increases the volume of feces in the intestine (intraluminal volume) which naturally stimulates intestinal motility. Furthermore, Mg2+ ions cause the release of cholecystokinin (CCK), which results in intraluminal accumulation of water, electrolytes, and increased intestinal motility. Some sources claim that the hydroxide ions themselves do not play a significant role in the laxative effects of milk of magnesia, as basic solutions (i.e., solutions of hydroxide ions) are not strongly laxative, and non-basic Mg2+ solutions, like MgSO4, are equally strong laxatives, mole for mole.[4] Only a small amount of the magnesium from magnesium hydroxide is usually absorbed by the intestine (unless one is deficient in magnesium). However, magnesium is mainly excreted by the kidneys so long-term, daily consumption of milk of magnesia by someone suffering from kidney failure could lead in theory to hypermagnesemia. Unabsorbed drug is excreted in feces; absorbed drug is excreted rapidly in urine.
References
[1]Margarete Seeger; Walter Otto; Wilhelm Flick; Friedrich Bickelhaupt; Otto S. Akkerman. "Magnesium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_595.pub2.
[2]Aileen Gibson and Michael Maniocha White Paper: The Use Of Magnesium Hydroxide Slurry For Biological Treatment Of Municipal and Industrial Wastewater, August 12, 2004
[3]Rothon, RN (2003). Particulate Filled Polymer Composites. Shrewsbury, UK: Rapra Technology. pp. 53–100.
[4]Tedesco FJ, DiPiro JT (1985). "Laxative use in constipation". Am. J. Gastroenterol. 80 (4): 303–9. PMID 2984923.
You may like
Related articles And Qustion
See also
Lastest Price from Magnesium hydroxide manufacturers

US $0.00-0.00/KG2025-12-12
- CAS:
- 1309-42-8
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $10.00/kg2025-04-21
- CAS:
- 1309-42-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt