Tungsten trioxide: photocatalytic properties and applications in antibiotics removal
General Description
Tungsten trioxide (WO3) exhibits excellent photocatalytic properties, making it suitable for the degradation of antibiotics in wastewater. It has a narrow bandgap, good electron mobility, and resistance to corrosion. WO3 can generate hydroxyl radicals, which are crucial for pollutant degradation. The addition of Pt as a cocatalyst enhances its activity by producing·OH radicals. WO3/g-C3N4 composite photocatalysts utilize electron holes in the degradation process. WO3 has been proven effective in degrading antibiotics like tetracycline, florfenicol, amoxicillin, and ciprofloxacin. Its application varies depending on catalyst composition, reaction conditions, and specific antibiotics. Further research is needed to optimize and scale up this technology for practical wastewater treatment. WO3-based photocatalysis shows promise for efficient antibiotic degradation.

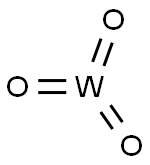
Figure 1. Tungsten trioxide
Photocatalytic properties
Tungsten trioxide is a widely used metal oxide with high research value, particularly in photochromic and photocatalytic applications. It exhibits a typical n-type semiconductor behavior with a crystal structure similar to distorted rhenium trioxide (ReO3). The octahedral structure of WO6 units, consisting of one tungsten atom at the center and six oxygen atoms at the apex, forms a symmetrical cubic structure. The synthesis conditions can cause distortions and affect the physical and chemical properties of WO3. With a bandgap of 2.4-2.8 eV, WO3 has a narrower bandgap compared to other semiconductors, enabling enhanced photocatalytic oxidation at the valence band edge potential. It also possesses medium hole diffusion length, good electron mobility, and resistance to corrosion in acid electrolytes. Additionally, WO3 exhibits low toxicity, wide availability, and cost-effectiveness, making it an efficient visible light active photocatalyst. China is the largest producer and exporter of tungsten products globally, and WO3 stands out among tungsten-based oxides due to its stable crystal structure, resistance to photo corrosion, and excellent recycling performance. These characteristics make it highly suitable for photocatalytic degradation of antibiotics in water bodies. 1
Applications in antibiotics removal
Tungsten trioxide has shown great potential in the application of antibiotics removal through photocatalysis. Several studies have been conducted to explore its effectiveness in degrading different antibiotics. Pt/WO3 nanomaterials exhibited higher photodegradation rates of tetracycline (TC) compared to WO3 alone under visible-light irradiation, thanks to the role of Pt as a cocatalyst. TC degradation experiments using 2% Pt/WO3 resulted in significant degradation of TC and removal of total organic carbon (TOC). WO3 nano sheets have been used as catalysts for the degradation of florfenicol (FLO). The unique hollow-sphere structure of WO3/g-C3N4 composite photocatalyst enhances the degradation efficiency of TC hydrochloride and ceftiofur sodium under visible light. The synthesis of WO3-center 0.33H2O/Ag2MoO4 composite showed promising results in degrading levofloxacin. The photocatalytic degradation of amoxicillin (AMO) was studied using WO3 as a catalyst, and optimal conditions for AMO degradation were determined. Fiber nanostructures in layered porous WO3/CdWO4 tubes demonstrated excellent photocatalytic degradation performance of ciprofloxacin (CIP) and TC. Cu-ion-loaded WO3 (Cu-WO3) proved to be an effective catalyst in the decomposition of TC in wastewater, especially under strongly alkaline conditions. WO3/BiOBr composite photocatalyst exhibited higher removal rates of CIP hydrochloride compared to BiOBr and WO3 alone. The use of photocatalyst tunneling nanotubes (TNTs)/WO3 resulted in significant degradation of sulfamethazine (SMZ), surpassing the degradation rates of TNTs and P25. In summary, the application of Tungsten trioxide in antibiotics removal through photocatalysis has shown promising results. Its effectiveness varies depending on the specific antibiotics and catalyst composition used, as well as the reaction conditions such as irradiation time and pH. Further research is needed to optimize and scale up these processes for practical applications in wastewater treatment. 2
Photocatalytic oxidation is an environmentally friendly technology that can degrade organic pollutants in wastewater. Tungsten trioxide is a multifunctional material used as a photocatalyst. WO3 can produce hydroxyl radicals, which are important for the degradation of pollutants. The addition of Pt as a cocatalyst enhances the photocatalytic activity of WO3 by generating·OH radicals through the reaction of excited electrons with oxygen. The degradation mechanism involves the generation of superoxide and hydroxyl radicals, which react with the pollutants to degrade them. WO3/g-C3N4 composite photocatalyst also utilizes electron holes in the degradation process. Overall, WO3-based photocatalysis shows promise for the efficient degradation of various antibiotics in wastewater. 3
Reference
1. Li S, Hu S, Zhang J, Jiang W, Liu J. Facile synthesis of Fe(2)O(3) nanoparticles anchored on Bi(2)MoO(6) microflowers with improved visible light photocatalytic activity. J Colloid Interface Sci, 2017, 497:93-101.
2. Yuju S, Xiujuan T, Dongsheng S, Zhiruo Z, Meizhen W. A review of tungsten trioxide (WO3)-based materials for antibiotics removal via photocatalysis. Ecotoxicol Environ Saf, 2023, 259:114988.
3. Katsumata H, Oda Y, Kaneco S, et al. Photocatalytic activity of Ag/CuO/WO3 under visible-light irradiation.RSC Advances, 2013, 3(15):5028.
Related articles And Qustion
See also
Lastest Price from Tungsten trioxide manufacturers
US $0.00-0.00/KG2025-12-13
- CAS:
- 1314-35-8
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00/KG2025-04-15
- CAS:
- 1314-35-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg