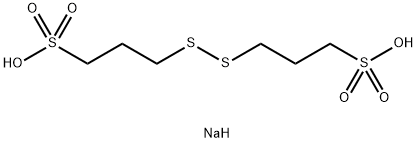
Bis-(sodium sulfopropyl)-disulfide: properties, applications and safety
General Description
Bis-(sodium sulfopropyl)-disulfide (SPS) is a versatile compound used in scientific research and industrial applications. It appears as a white to off-white powder. SPS is highly soluble in water, forming a clear and colorless solution. With sulfonic acid groups and a disulfide bridge in its structure, it has reactive properties towards nucleophiles, making it suitable for covalent modification of biomolecules. However, it remains stable under normal laboratory conditions. SPS finds applications in copper surface studies, electrochemical experiments, and is effective in synergy with polyethylene glycol. Safety precautions include avoiding skin contact, wearing protective equipment, and proper storage and disposal.

Figure 1. Bis-(sodium sulfopropyl)-disulfide
Properties
Bis-(sodium sulfopropyl)-disulfide is a chemical compound widely used in scientific and industrial applications. This compound possesses several important properties. In terms of physical properties, SPS has a molecular weight of 354.4 g/mol and appears as a white to off-white powder. It exhibits high solubility in water, leading to the formation of a clear and colorless solution. The melting point of SPS is approximately 210-215°C. Chemically, SPS contains two sulfonic acid groups (-SO3H) and a disulfide bridge (-S-S-) in its structure. These functional groups make SPS reactive towards nucleophiles, allowing it to be used for covalent modification of biomolecules. However, under normal laboratory conditions, SPS is stable. It may decompose when exposed to strong acids or bases. Overall, Bis-(sodium sulfopropyl)-disulfide possesses desirable physical and chemical properties that make it suitable for various applications in scientific research and industrial processes. 1
Applications
Bis-(sodium sulfopropyl)-disulfide is a functional additive that has been studied in various applications involving copper surfaces. In a study, interactions of SPS, along with other additives including MPS and Cl, on a copper electrodeposited layer were investigated using TOF-SIMS and CV techniques. The results demonstrated that the surface coverage of Cl and MPS depended on the applied overpotential and the concentrations of Cl, SPS, or MPS in the solution. A detailed discussion on the mechanism of fragment formation, such as CH2SO3-, C3H5SO3-, CuSC3H6SO3-, and CuS-, was proposed and their assignment to the gauche or trans conformation was discussed. The study also proposed a mechanism for the incorporation and re-adsorption of MPS onto a copper surface under different electrochemical conditions, both with and without chloride ions, and examined its impact on electrochemical properties. It was found that the presence of chloride ions, as well as the ratio of gauche/trans MPS molecules and the ratio of chloride/thiols, had a significant influence on the accelerating abilities. Additionally, the interactions of SPS and polyethylene glycol (PEG) in the presence of chloride ions were investigated. The surface coverages of PEG, thiolate, and chloride were estimated and their electrochemical suppressing/accelerating abilities were discussed. The conformational influence of thiolate molecules and the C-C and C-O regions of PEG on electrochemical properties was also explored. It was observed that the addition of SPS slightly reduced the contribution of the hydrophobic interaction between PEG and chloride ions, while significantly diminishing the contribution of Cu-PEG adducts. Bis-(sodium sulfopropyl)-disulfide and PEG exhibited significant synergy through co-adsorption. 2
Safety
In terms of health hazards, bis-(sodium sulfopropyl)-disulfide can cause sensitization and may cause allergic skin reactions. It is important to avoid breathing in dust, fumes, or vapors, and if skin irritation occurs, medical advice should be sought. In case of accidental ingestion, one should rinse the mouth and consult a physician. During firefighting, it is crucial to wear appropriate protective clothing and a self-contained breathing apparatus, as SPS releases toxic fumes when exposed to fire. Contaminated fire extinguishing water should be collected separately and not discharged into drains. When handling SPS, it is recommended to avoid direct contact with skin, eyes, and clothing. Proper ventilation should be provided, and contaminated clothing should be washed before reuse. SPS should be stored at temperatures between 2°C and 8°C in a tightly closed container, away from incompatible substances. To ensure personal protection, respiratory protection, chemical-resistant gloves, protective eyeglasses or goggles, and appropriate clothing should be worn. Mechanical exhaust, safety showers, and eye showers should also be available in the workplace. Overall, the safety precautions for bis-(sodium sulfopropyl)-disulfide include careful handling, proper personal protective equipment, and complying with regulations and disposal guidelines. 3
Reference
1. PubChem. Compound Summary: Bis-(sodium sulfopropyl)-disulfide. National Library of Medicine CID:117948
2. Mroczka R, Słodkowska A, Ładniak A. Studies of Bis-(Sodium-Sulfopropyl)-Disulfide and 3-Mercapto-1-Propanesulfonate on/into the Copper Electrodeposited Layer by Time-of-Flight Secondary-Ion Mass Spectrometry. Molecules. 2022 Nov 22;27(23):8116.
3. Safety Data Sheet: Bis-(Sodium-Sulfopropyl)-Disulfide. Biosynth, 2021, Biosynth catalog no. FD10908.
You may like
Related articles And Qustion
Lastest Price from Bis-(sodium sulfopropyl)-disulfide manufacturers

US $0.00/kg2025-08-26
- CAS:
- 27206-35-5
- Min. Order:
- 1kg
- Purity:
- 99%MIN
- Supply Ability:
- 20tons

US $10.00/KG2025-04-21
- CAS:
- 27206-35-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt