Triethylsilane: General description,Application and Production
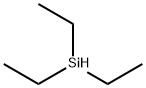
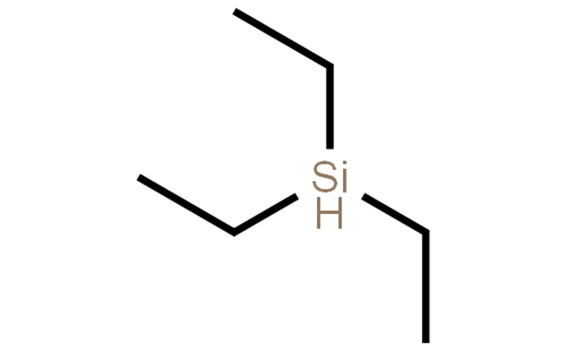
Triethylsilane, with the chemical formula C6H16Si, is an organosilicon compound. It is colorless and transparent liquid, soluble in non-polar solvents and flammable. Triethylsilane has properties including high stability, high addition reactivity, relatively low surface tension and viscosity, and excellent protective properties, so it is widely used in silicone rubber, silicone gel, ink, water repellent, interface agent and feed additive. In addition, it can also be used as a precursor for vapor deposition (CVD) and chemical vapor deposition (PECVD) reactions[1,2].

Figure1 Triethylsilane - pharmaceutical raw materials both for vet and human
Application and Pharmacology
Triethylsilane has a variety of useful applications in organic chemistry, but it is not commonly used in medicine due to its potential hazards. The role of triethylsilane in applications includes:
1.As a cross-linking agent in silicone rubber and silicone gels, it can improve the hardness, strength, heat resistance and chemical resistance of materials[3].
2.As a dispersant and thickener in inks and coatings, it can improve the fluidity and uniformity of coatings and form a good protective layer.
3.As a water repellent and interface agent, it can increase the surface tension of materials, improve moisture resistance and reduce the coefficient of friction.
4.As an additive in the production of food and feed, it can improve the quality and shelf life of products.
5.In scientific research, it can be used as a precursor for chemical vapor deposition (PECVD), for the preparation of nanomaterials, thin films and as a representative of organosilicon compounds, triethylsilane has a wide range of applications in numerous fields.
6.Silicone rubbers and silicone gels: triethylsilane can be co-polymerized with other silicone compounds to form silicone rubbers and silicone gels to improve their physical and chemical properties for the manufacture of seals, medical devices and other products.
7.Inks: triethylsilane can be used as viscosity modifiers or emulsifiers to improve ink flow and printing quality.
8.Water repellents: triethylsilane can prevent materials from water erosion and is commonly used in building materials, coatings and textiles, etc.
9.Surfactants: triethylsilane can reduce the surface tension and viscosity of liquids and improve the compatibility and dispersion of substances between different phases.
10.Pharmaceutical and bioengineering: triethylsilane can be used for drug delivery and storage, as well as in areas such as bioimaging and cell culture.
In pharmacology, some studies have shown that triethylsilane has antioxidant, anti-inflammatory and antibacterial effects, which can prevent or alleviate some diseases such as liver fibrosis, diabetes, and skin inflammation. In addition, triethylsilane can enhance the permeability of therapeutic drugs and improve their efficacy. It should be noted that more research and validation of triethylsilane is still needed for its clinical application effectiveness and safety in pharmacology. It is not used as a drug in medicine. However, it has been shown to have some neuroprotective properties in animal studies. It can protect neurons from oxidative stress and reduce the risk of brain damage.
Synthesis
1.Trichlorosilane and ethyl lithium reaction method: Trichlorosilane is reacted with ethyl lithium under inert atmosphere to produce triethylsilane and lithium chloride. The reaction equation is as follows:
SiCl3 + 3C2H5Li → Si(C2H5)3 + 3LiCl
2.Reaction method of triethylchlorosilane and ethanol: react triethylchlorosilane and ethanol under alkaline conditions to produce triethylsilane and hydrogen chloride [4]. The reaction equation is as follows:
Si(C2H5)3Cl + 3C2H5OH → Si(C2H5)3 + 3HCl + 3C2H5OH
Both of the above methods can prepare high purity triethylsilane, but the former requires the use of highly toxic ethyl lithium, which is more difficult to operate; the latter requires the reaction under alkaline conditions, which is corrosive to the equipment and environment. Therefore, the specific method to be used needs to be considered according to the actual situation.
Toxicity and safety
Triethylsilane has very low toxicity and is a relatively safe chemical. It is a non-volatile liquid, not volatile, not easy to burn naturally, and has low surface tension, viscosity and surface energy, which can play an excellent role in wetting, adhesion and modification in coatings and cosmetics. It is flammable and should be handled with care. It can also be harmful if ingested, inhaled or comes into contact with the skin or eyes. It should only be used in a well-ventilated area, with appropriate protective equipment. However, triethylsilane is a flammable liquid and will burn and explode when it comes into contact with fire sources. Meanwhile, triethylsilane will decompose and produce toxic gas under high temperature for a long time, so avoid high temperature during operation and storage.In general, triethylsilane is a relatively safe chemical, but care should still be taken to operate safely and avoid sources of ignition when using and storing it[5].
Reference
1. Trost, B. M.; Ball, Z. T. J. Am. Chem. Soc., 2001, 123, 12726.
2. Sato, A.; Kinoshita, H.; Shinokubo, H.; Oshima, K. Org. Lett., 2004, 6, 2217.
3. Liu, Yang; Yamazaki, Shoko; Yamabe, S. J. Org. Chem., 2005, 70, 556.
4. Rubin, M.; Schwier, T.; Gevorgyan, V.; J. Org. Chem., 2002, 67, 1936.
5. Asao, N.; Ohishi, T.; Sato, K.; Yamamoto, Y. J. Am. Chem. Soc., 2001, 123, 6931.
Related articles And Qustion
See also
Lastest Price from Triethylsilane manufacturers

US $0.00-0.00/KG2025-05-21
- CAS:
- 617-86-7
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS

US $0.00/KG2025-05-19
- CAS:
- 617-86-7
- Min. Order:
- 1000KG
- Purity:
- 99
- Supply Ability:
- 20000