The Versatile Applications and Characteristics of Triethylsilane in Modern Chemistry
Introduction
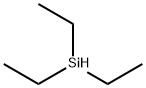
Triethylsilane is a versatile organosilicon compound known for its unique properties and applications in various industries. This colorless liquid is characterized by its low viscosity and high reactivity, making it an excellent choice as a reducing agent in organic synthesis. Researchers and industry professionals often utilize triethylsilane in the preparation of silanes and siloxanes and in synthesising pharmaceuticals and agrochemicals[1].
Chemical property
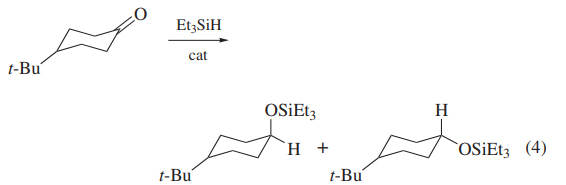
Triethylsilane serves as an exemplar for organosilicon hydride behavior as a mild reducing agent. It is frequently chosen as a synthetic reagent because of its availability, convenient physical properties, and economy relative to other organosilicon hydrides, which might otherwise be suitable for effecting specific chemical transformations. Triethylsilane is a colorless liquid. It is insoluble in H2O and hydrocarbons, halocarbons, and ethers[2].
Uses
Reductive amination between aldehydes or ketones and amines occurs smoothly within the hydrophobic cores of nano micelles in water. A broad range of substrates can be converted under mild conditions in the presence of 0.20 mol % Pd/C and triethylsilane, leading to high chemical yields of the desired secondary and tertiary amines.
A simple iron- and silyl chloride-catalyzed reductive etherification enables the preparation of symmetrical and nonsymmetrical ethers from various aldehydes and ketones in the presence of triethylsilane as a reducing agent and catalytic amounts of iron(III) oxo acetate and chloro(trimethyl)silane. The reactions can be carried out at ambient temperatures using ethyl acetate as the solvent.
Facile reductive etherification of carbonyl compounds can be conveniently performed by reaction with triethylsilane and alkoxytrimethylsilane catalyzed by iron(III) chloride. The reduced alcohols' corresponding alkyl ethers, including benzyl and allyl ethers, were obtained in good to excellent yields under mild reaction conditions.
The combination of the tris-4-bromophenylamminium radical cation, commonly known as magic blue (MB•+), and triethyl silane mediates a mild OtBu deprotection. Magic blue catalytically facilitates the cleavage of the C-O bond in tert-butyl carbamates, carbonates, esters, and ethers in a high isolated yield under mild conditions, and sacrificial triethyl silane accelerates the reaction.
Precautions
Triethylsilane is physically very similar to comparable hydrocarbons. It is a flammable, but not pyrophoric, liquid. As with all organosilicon hydrides, it can release hydrogen gas upon storage, particularly in the presence of acids, bases, or fluoride-releasing salts. Proper precautions should be taken to vent possible hydrogen buildup when opening vessels where triethylsilane is stored.
References
[1]Connelly S J, Kaminsky W, Heinekey D M. Structure and solution reactivity of (triethylsilylium) triethylsilane cations[J]. Organometallics, 2013, 32(24): 7478-7481.
[2]Anderson H H. Reactions of triethylsilane and diethylsilane with inorganic halides and acids[J]. Journal of the American Chemical Society, 1958, 80(19): 5083-5085.
Related articles And Qustion
Lastest Price from Triethylsilane manufacturers

US $0.00-0.00/KG2025-05-21
- CAS:
- 617-86-7
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS

US $0.00/KG2025-05-19
- CAS:
- 617-86-7
- Min. Order:
- 1000KG
- Purity:
- 99
- Supply Ability:
- 20000