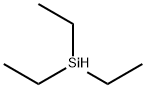
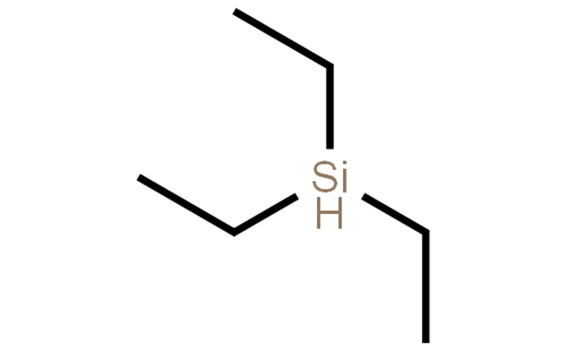
How does the reduction mechanism of triethylsilane and trans fatty acids work?
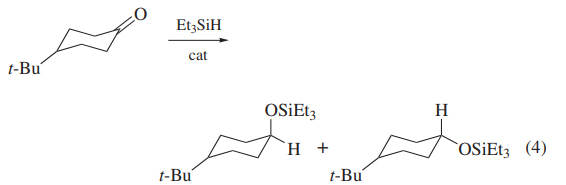
Triethylsilane is a commonly used reducing agent in organic reactions for a wide range of functional groups, including alcohols, aldehydes, ketones, and amides. It is particularly suitable for the reduction of carbonyl and imine functional groups. It is usually used in combination with trifluoroacetic acid (TFA) as a co-catalyst. The reduction mechanism of triethylsilane and TFA involves the transfer of hydride ions from the silane to the substrate, resulting in the formation of a silylene ether intermediate. This intermediate can then react with TFA to form a stable carbon ion, which can then be protonated to produce the reduction product. In the reduction reaction, the triethylsilane first needs to be activated, which is usually achieved by sodium or potassium hydroxide. During the activation process, the silicon-hydrogen bonds in the triethylsilane are broken, which enhances its reducing ability.
Related articles And Qustion
Lastest Price from Triethylsilane manufacturers

US $0.00-0.00/KG2025-05-21
- CAS:
- 617-86-7
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS

US $0.00/KG2025-05-19
- CAS:
- 617-86-7
- Min. Order:
- 1000KG
- Purity:
- 99
- Supply Ability:
- 20000