Triethylenediamine: Application, synthesis and bioactivity
General description
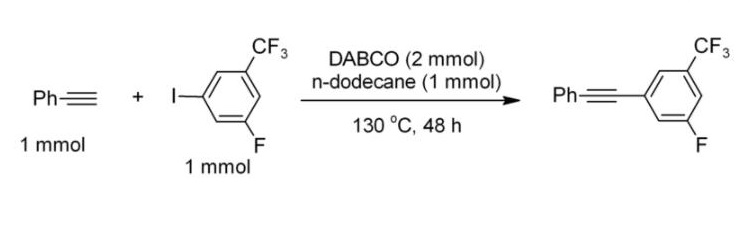
Triethylenediamine(DABCO) is a bicyclic organic compound with the formula N2(C2H4)3. This colorless solid is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent in polymerization and organic synthesis. The pKa of [HDABCO]+ (the protonated derivative) is 8.8, which is almost the same as ordinary alkylamines. The nucleophilicity of the amine is high because the amine centers are unhindered. It is sufficiently basic to promote C–C coupling of terminal acetylenes, for example, phenylacetylene couples with electron-deficient iodoarenes. Its appearance is as follows:

Figure 1 Appearance of Triethylenediamine.
Synthesis
The Triethylenediamine can be synthesized by the previous work [1]. The reactions were carried out in a down-flow, fixed-bed silica reactor at atmospheric pressure and 300-400 °C. Prior to each run of the reactor, the catalyst (3 g, 0.3-0.7 mm) was calcined for 3 h in a flow of dry air and then cooled to the reaction temperature in a flow of N2. Aqueous EDA solution (EDA/H2O molar ratio) was introduced into the reactor via an ISCO pump (Model 500D, USA) with a flow rate of 3 mL/h. The temperature was measured by a thermocouple which was located in the catalyst bed. The products were cooled by an ice-cooled water bath and collected in the receiver, and were analyzed on SP 6890 gas chromatograph (SP 6890 with a FID detector, 30 m, CP-Sil-5 Chrompack column, id: 0.25 mm, coating 0.25 lm, column temperature 150 °C, injector temperature 200 °C and FID temperature 250 °C). The products were identified by GC-MS (Varianinc GC/MS 2100). The yield of triethylenediamine was 64%.
Application
Triethylenediamine is used as a base-catalyst for formation of polyurethane from alcohol and isocyanate functionalized monomers and pre-polymers. In addition, it can also be used in Baylis-Hillman and Morita-Baylis-Hillman reactions of aldehydes and unsaturated ketones and aldehydes. As an unhindered amine, it is a strong ligand and Lewis base. It forms a crystalline 2:1 adduct with hydrogen peroxide and sulfur dioxide [2]. Triethylenediamine can be used to synthesize doubly-charged styrenic monomers. These ionic monomers allow synthesis of polyelectrolytes and ionomers with two cyclic quaternary ammonium cations on each ionic pendant group [3]. Triethylenediamine and related amines are quenchers of singlet oxygen and effective antioxidants [4], and can be used to improve the lifetime of dyes. This makes triethylenediamine useful in dye lasers and in mounting samples for fluorescence microscopy (when used with glycerol and PBS) [5]. Triethylenediamine can also be used to demethylate quaternary ammonium salts by heating in dimethylformamide (DMF).
Bioactivity
Standard MIC assays were performed to measure the antifungal activities of Triethylenediamine against C. albicans according to CLSI guidelines. The MIC90 value of the Triethylenediamine ranged from 2 to 4 μg/mL. No growth was observed from plated samples at the MIC level or above, indicating that Triethylenediamine exhibit fungicidal activity. Triethylenediamine was used to investigate antifungal activity against several Candida species, including C. albicans, C. dubliniensis, C. glabrata, C. parapsilosis, and C. tropicalis, and to compare the activity with that of traditional antifungal azoles, clotrimazole and fluconazole. Triethylenediamine exhibited antifungal activity against all Candida species, with a MIC90 range of 2 to 4 μg/ml, similar to that of fluconazole. While, as expected, clotrimazole MIC values were lower, both azole drugs were confirmed as static inhibitors of growth, while Triethylenediamine exhibited fungicidal activity [6].
Triethylenediamine was tested for cytotoxicity against periodontal ligament (PDL) cells, gingival fibroblasts (GF), or primary oral epithelial (EPI) cells. Cell cultures were exposed to Triethylenediamine or a standard cytotoxicity control compound, BisGMA, for 24 h and assessed for cell survival. Triethylenediamine exhibited toxicity similar to or greater than that of the BisGMA positive control, reaching 50% cytotoxicity in the range of 2.0 to 8.0 μg/mL, depending on the compound and cell type [6].
References
[1]Zhao et al. Synthesis of 1,4-Diazabicyclo[2.2.2]octane over Pretreated Titanium Silicalite-1 Catalyst. Catalysis Letters (2008), 126(3-4), 383-387.
[2]Ludovic et al. Preparation of DABSO from Karl-Fischer Reagent. Org. Synth. 2013, 90, 301.
[3]Zhang, et al. Styrenic DABCO salt-containing monomers for the synthesis of novel charged polymers. Polymer Chemistry (2016), 7 (20): 3370–3374.
[4]Ouannes et al. Quenching of singlet oxygen by tertiary aliphatic amines. Effect of DABCO (1,4-diazabicyclo[2.2.2]octane). Journal of the American Chemical Society. (1968), 90 (23): 6527–6528.
[5]Valnes et al. Retardation of immunofluorescence fading during microscopy. Journal of Histochemistry and Cytochemistry. (1985), 33 (8): 755–761.
[6]Herman et al. Synthesis, Antifungal Activity, and Biocompatibility of Novel 1,4-Diazabicyclo[2.2.2]Octane (DABCO) Compounds and DABCO-Containing Denture Base Resins. Antimicrobial Agents and Chemotherapy 2017, 61(4), 234-239.
You may like
Related articles And Qustion
See also
Lastest Price from Triethylenediamine manufacturers

US $0.00/kg2025-09-18
- CAS:
- 280-57-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS

US $1.00/KG2025-04-21
- CAS:
- 280-57-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt