What is Triethylenediamine?
Aug 21,2020
Introduction
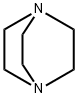
DABCO (1,4-diazabicyclo[2.2.2]octane) is an organic compound with the formula N2(C2H4)3. This colorless solid is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent in polymerization and organic synthesis.
Quinuclidine has a similar structure, with one of the nitrogen atoms replaced by a carbon atom.Crystalline 1,4-Diazabicyclo[2.2.2]octane is a high-purity triethylenediamine (TEDA). This highly basic amine is used as a catalyst and reagent in polymerization and organic synthesis. DABCO is an effective antioxidant and a quenchers of singlet oxygen that can be used as a stabilizer of dye solutions which have not been oxygen degassed.
Uses
An anti-fade reagent shown to scavenge free-radicals due to flurochrome excitation. It is used for acetylation of alcohols using Acetylating Reagents and Base Catalysts.
Purification Methods
DABCO crystallises from 95% EtOH, pet ether or MeOH/diethyl ether (1:1). Dry it under vacuum over CaCl2 and BaO. It can be sublimed in vacuo, and readily at room temperature. It has also been purified by removal of water during azeotropic distillation of a *benzene solution. It is then recrystallised twice from anhydrous diethyl ether under argon, and stored under argon [Blackstock et al. J Org Chem 52 1451 1987]. [Beilstein 23/3 V 487.]
Reactions and applications
The pKa of [HDABCO]+ (the protonated derivative) is 8.8, which is almost the same as ordinary alkylamines. The nucleophilicity of the amine is high because the amine centers are unhindered. It is sufficiently basic to promote C–C coupling of terminal acetylenes, for example, phenylacetylene couples with electron-deficient iodoarenes.
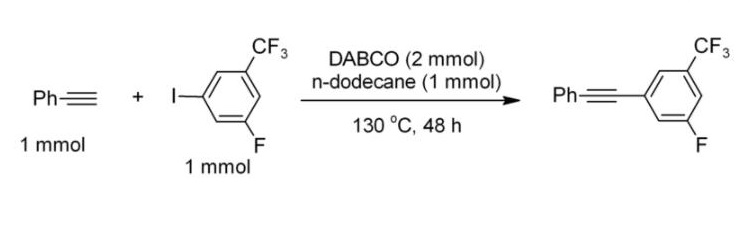
Example of a DABCO-catalysed C-C coupling Catalyst.
DABCO is used as a base-catalyst for:
formation of polyurethane from alcohol and isocyanate functionalized monomers and pre-polymers.
Baylis-Hillman and Morita-Baylis-Hillman reactions of aldehydes and unsaturated ketones and aldehydes.
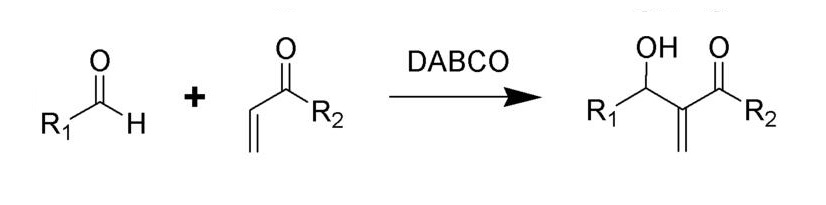
The scheme of Baylis Hilman reaction.
Lewis base
As an unhindered amine, it is a strong ligand and Lewis base. It forms a crystalline 2:1 adduct with hydrogen peroxide[6] and sulfur dioxide.
Ionic monomer synthesis
DABCO can be used to synthesize doubly-charged styrenic monomers. These ionic monomers allow synthesis of polyelectrolytes and ionomers with two cyclic quaternary ammonium cations on each ionic pendant group.
Quencher of singlet oxygen
DABCO and related amines are quenchers of singlet oxygen and effective antioxidants, and can be used to improve the lifetime of dyes. This makes DABCO useful in dye lasers and in mounting samples for fluorescence microscopy (when used with glycerol and PBS). DABCO can also be used to demethylate quaternary ammonium salts by heating in dimethylformamide (DMF).
You may like
What you need to know about ceramides?
Apr 17, 2024
Related articles And Qustion
See also
Lastest Price from Triethylenediamine manufacturers
Triethylenediamine

US $0.00/kg2025-09-18
- CAS:
- 280-57-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS
Triethylenediamine/TEDA

US $1.00/KG2025-04-21
- CAS:
- 280-57-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt