The uses of tert-Butyl acetoacetate
Tert-butyl acetoacetate(t -BAA) is a colorless liquid, stable under normal temperature and pressure, avoiding strong oxidant contact.tert -Butyl acetoacetate is a cheap and easy to store commercial reagent. Reaction demonstrated that the more hindered tert-butyl acetoacetate is more reactive than the more commonly used methyl or ethyl analogs. tert-butyl acetoacetate is widely employed as an acetoacetylating reagent.

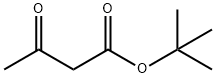
Fig 1. Chemical structure formula and three-dimensional structure of tert-Butyl acetoacetate
Uses
tert-Butyl acetoacetate is a reagent used to acetoacetylate hydroxyl-bearing coatings resins, such as polyesters, acrylics, cellulosics, and epoxies. tert-Butyl acetoacetate has also found use as an acetoacetylating reagent in pharmaceutical, agrichemical, and pigment applications. tert-Butyl acetoacetate acetoacetylates alcohols and amines in high yields.
tert-Butyl acetoacetate was refluxed for 3 hr over anhydrous CaCl, and distilled through a Vigreux column under reduced pressure, the middle fraction being collected. The copper complex precipitated upon mixing aqueous solutions of CuSO4, and t-butyl acetoacetate and NaOH. The green product has collected on a filter. Thoroughly washed with water, and air-dried. The dried product was then recrystallized from n-hexane twice and placed in a vacuum dessicator over P:O for 3 days[1].
Reaction of various nucleophiles with tert-butyl acetoacetate (t-BAA, la) is shown to be a convenient method for the preparation of a wide variety of acetoacetic acid derivatives. This material can be used to prepare acetoacetates and acetoacetamides from a wide variety of alcohols and amines. Reaction of la with an unhindered primary amine such as n-heptylamine under standard conditions gives unwanted byproducts due to the formation of the enamines. Formation of these byproducts can be minimized by dilution and/or altering the mode of addition[2].
Highly enantioselective Mannich reactions of imines bearing a benzothiazole moiety with tert-butyl acetoacetate, catalyzed by a cinchona-based squaramide organocatalyst have been developed. The corresponding benzothiazole β-keto ester derivatives were obtained in high yields (up to 99%) and with excellent enantioselectivities (up to 98% ee)[3].
The acetoacetylation mechanism using tert-butyl acetoacetate is distinctly different from that of typical transesterification reactions and allows for the acetoacetylation of polyesters without altering the backbone structure. Furthermore, this reaction is 15 to 20 times faster than reaction with methyl acetoacetate or ethyl acetoacetate.tert-butyl acetoacetate is equally effective in acetoacetylating primary and secondary hydroxyls.
References
1.Witzeman J S , Nottingham W D . Transacetoacetylation with tert-butyl acetoacetate: synthetic applications[J]. Journal of Organic Chemistry, 1991, 56(5):1713-1718.
2.Garito A F , Wayland B B . Thermodynamics and magnetic resonance of five-coordinate copper tert-butyl acetoacetate-pyridine adducts in cyclohexane[J]. Journal of the American Chemical Society, 1969, 91(4):866-872.
3.He H, Du D. ChemInform Abstract: Highly Enantioselective Mannich Reactions of Imines with tert‐Butyl Acetoacetate Catalyzed by Squaramide Organocatalyst.[J]. 2015, 45(41):637-643.
See also
Lastest Price from tert-Butyl acetoacetate manufacturers

US $99.00-45.00/kg2025-04-21
- CAS:
- 1694-31-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton

US $30.00-10.00/KG2025-04-15
- CAS:
- 1694-31-1
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg