The uses of 4-Methoxyphenylboronic acid
4-Methoxyphenylboronic acid is a reagent used in the preparation of various biological inhibitors.
Uses
4-Methoxyphenylboronic acid is a reagent used for Suzuki-Miyaura cross-coupling reactions1;Pd-catalyzed direct arylation;Highly effective synthesis using palladium-catalyzed arylation Suzuki-Miyaura cross-coupling in water;Palladium-catalyzed stereoselective Heck-type reaction;Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence;Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides;Ruthenium catalyzed direct arylation;Rh-catalyzed asymmetric conjugate addition. Studies on 4-methoxyphenylboronic acid is a thioformamide palladium complex as a Suzuki coupling catalyst. 4-methoxyphenylboronic acid push-pull arylvinyldiazine chromophore with photophysical properties.
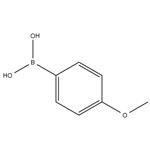
Fig 1. Chemical structure formula and three-dimensional structure of 4-Methoxyphenylboronic acid
4-Methoxybenzeneboronic acid is used for Suzuki-Miyaura cross-coupling reactions, Pd-catalyzed direct arylation, Highly effective synthesis using palladium-catalyzed arylation Suzuki-Miyaura cross-coupling in water, Palladium-catalyzed stereoselective Heck-type reaction, Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence, Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides, Ruthenium catalyzed direct arylation, Rh-catalyzed asymmetric conjugate addition, Ligand-free copper-catalyzed coupling[1-3].
Improved syntheses of optically pure(-)- and (+)- morinol C, and (-)- and (+)-morinol D were achieved by employing the Mizoroki-Heck reaction to construct the cinnamyl moiety. The protective group of the alkene substrate affected the yield of this key reaction. The reaction with a combination of the acetate-protected olefin and 4-methoxyphenylboronic acid gave the best result producing morinol C and D.All stereoisomers of morinol C and D showed cytotoxic activity, with (R,R)-morinol C showing the highest antibacterial activity[4].
Simultaneous chemical vapor deposition (CVD) of graphene and “in-situ” phosphorous or boron doping of graphene was accomplished using Triphenylphosphine (TPP) and 4-Methoxyphenylboronic acid. The TPP and 4-MPBA molecules were sublimated and supplied along with CH4 molecules during graphene growth at atmospheric pressure. The grown graphene samples were characterized using Raman spectroscopy. Phosphorous and boron presence in phosphorous and boron doped graphene was confirmed with Auger electron spectroscopy. The possibility of obtaining phosphorous and boron doped graphene using solid-source molecule precursors via CVD can lead to an easy and rapid production of modified large area graphene[5].
Supramolecular assemblies of phenylboronic and 4-methoxyphenylboronic acids with 4,40-bipyridine and an assembly of phenylboronic acid with 1,2-bis(4-pyridyl)ethene, which were obtained due to the formation of O–H...N hydrogen bonds between hetero N-atoms and –B(OH)2 are reported. Further, a centrosymmetric cyclic C–H...O hydrogen bonding dimer is identified in the crystal structure of 4-methoxyphenylboronic acid[6].
References
1.S.A. Barker.; A.K. Chopra.; B.W. Hatt.; P.J. Somers. The interaction of areneboronic acids with monosaccharides . Carbohydrate Research. 1973, 26 (1), 33-40.
2.S.A. Barker, B.W. Hatt, P.J. Somers. The effect of areneboronic acids on the alkaline conversion of d-glucose into d-fructose. Carbohydrate Research . 1973, 26 (1), 41-53.
3.For an illustrative example of the silver oxide promoted Suzuki coupling with sensitive ɑ-halo enones, under extremely mild conditions, see: Org. Synth., 75, 69 (1997).
4.OGURA, Koji, SUGAHARA, Takuya, MARUYAMA, Masafumi. Improved Syntheses of Morinol C and D by Employing Mizoroki-Heck Reaction and Their Cytotoxic and Antimicrobial Activities[J]. Biosci Biotechnol Biochem, 74(8):1641-1644.
5.Ovezmyradov M , Magedov I V , Frolova L V , et al. Chemical Vapor Deposition of Phosphorous- and Boron-Doped Graphene Using Phenyl-Containing Molecules[J]. Journal of Nanoscience and Nanotechnology, 2015, 15(7):4883-4886.
6.Zotto A D , Amoroso F , Baratta W , et al. Very Fast Suzuki-Miyaura Reaction Catalyzed by Pd(OAc)2 under Aerobic Conditions at Room Temperature in EGME/H2O[J]. European Journal of Organic Chemistry, 2009, 2009(1):7.
You may like
Related articles And Qustion
See also
Lastest Price from 4-Methoxyphenylboronic acid manufacturers
US $0.00/kg2025-03-03
- CAS:
- 5720-07-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS
US $34.00-1.20/kg2024-04-08
- CAS:
- 5720-09-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available