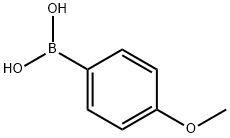
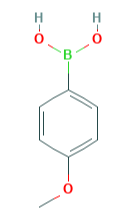
The application of 4-methoxyphenylboronic acid in organic synthesis
Introduction
4-Methoxyphenylboronic acid (Figure 1) can be used as a common organic reagent in Suzuki coupling reaction, and can be used to study the N-arylation of imidazole compounds and amines catalyzed by copper exchanged calcium fluorophosphate. Its appearance is white to grayish white powder with certain irritation. This paper mainly reports the application of 4-methoxyphenylboronic acid in organic synthesis.

4-Methoxyphenylboronic acid as boron sources
This work demonstrates the simultaneous chemical vapor deposition (CVD) growth and doping ofphosphorous- and boron-doped graphene using methane as the major source of carbon, with phenyl-containing molecules Triphenylphosphine (TPP) and 4-Methoxyphenylboronic acid (4-MPBA) as phosphorous and boron sources respectively. Both demonstrated variants of growth/doping are different from previously reported techniques. For P-doped graphene (graphene: P), the previously reported results have been obtained with graphene oxide,or not through a simultaneous process of growth and doping of graphene. For B-doped graphene, 4-methoxyphenylboronic acid was never used before as a doping source in graphene CVD growth process. Growth/doping of a few-layer P- and B-doped graphene was carried out at atmospheric pressure conditions. The structural properties of the graphene: P and graphene: B have been studied using Raman spectroscopy. Phosphorous and boron presence of graphene: P and graphene: B have been verified using Auger electron spectroscopy.
The simultaneous CVD growth of graphene along with phosphorous or boron doping was accomplished using phenyl-containing solid molecules as dopant materials.The phenyl-containing solid molecular precursors provide a possible method to obtain modified graphene for various applications, in particular for use in fuel cells and metalair batteries where the uniformity of graphene thickness is not critical.[1]
Suzuki coupling reaction with 4-methoxyphenylboronic acid
A series of 16 (hetero)aryl compounds based on coumarin and equol has been efficiently synthesized by exploring the palladium-catalyzed Suzuki cross-coupling reactions. Polyphenol based on coumarin (4-methyl-7-hydroxy coumarin) was initially converted to corresponding coumarin imidazylate and then subjected to Suzuki coupling reaction with 4-methoxyphenylboronic acid to obtain the coupled product. This modified approach was later developed into a one-pot methodology by directly reacting the polyphenol with 1,1-sulfonyldiimidazole (SDI) and boronic acid in situ to obtain the Suzuki coupled product in one step. Moreover, an array of (poly)phenols based on coumarin and equol were later converted to diverse (hetero)aryl compounds by this optimized step-economic protocol. The synthesized compounds were then subjected to the screening of their potential antioxidant activities by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. In our investigation, the compounds 4ah, 4eh, 4gh and 4hh exhibited promising antioxidant potential when compared to the reference standard, butylated hydroxytoluene (BHT). Structure activity relationship (SAR) studies revealed the importance of the presence of electron-donating substituents in enhancing the antioxidant activity of the synthesized compounds.[2]
Cross-coupling reaction
The major advancements in homogeneous transition metal catalysis from the 1970s, Mizoroki-Heck, Sonogashira-Hagihara, and Suzuki-Miyaura reactions, have become highly prominent and widely studied in organic synthesis. The Sonogashira reaction is particularly notable for enabling the cross-coupling of terminal alkynes with aryl or vinyl halides,making it a key method for forming essential C(sp2)−C(sp) bonds. This type of coupling reaction is especially valuable because of its broad applicability in pharmaceutical synthesis and natural products. The Chinese natural product selaginellin was isolated from Selaginella sinensis, and its derivatives contain the internal alkyne, which is used as a lead compound against type 2 diabetes. The natural product selaginpulvilins A-D shows the inhibitory activity against PDE4D2.The natural product chloropestolide shows antitumor and anti-HIV activities. The antibiotic natural product cuplin is isolated from culpepper, found in Virginia.
Bose et al. report a cross-coupling reaction between terminal alkyne and arylboronic acid to form the C-C bond in water microdroplets under mild, catalyst-free conditions. A solution containing phenylacetylene (50 μM) and 4-methoxyphenylboronic acid (50 μM) in a 4:1 (H2O: ACN) mixture was electrosprayed at +1.5 kV, and the reaction products were analyzed using a mass spectrometer (MS). Bose et al. observed C(sp2)-C(sp) coupling reaction products within milliseconds, which were characterized by tandem mass spectrometry (MS2). Based on in situ reaction studies and radical experiments, we determined the reaction mechanism for the coupling reaction. These approach offers a sustainable and environmentally benign pathway for the synthesis of internal aryl alkynes, making it a suitable building block for the preparation of various pharmaceutical drugs.[3]
References
1.Mekan Ovezmyradov, Magedov IV, Frolova LV, et al. Chemical Vapor Deposition of Phosphorous- and Boron-Doped Graphene Using Phenyl-Containing Molecules. J Nanosci Nanotechnol. 2015;15(7):4883-4886. doi:10.1166/jnn.2015.9827
2.Joy MN, Kovalev IS, Shabunina OV, Santra S, Zyryanov GV. Facile One-Pot Conversion of (poly)phenols to Diverse (hetero)aryl Compounds by Suzuki Coupling Reaction: A Modified Approach for the Synthesis of Coumarin- and Equol-Based Compounds as Potential Antioxidants. Antioxidants (Basel). 2024;13(10):1198. Published 2024 Oct 3. doi:10.3390/antiox13101198
3.Bose S, Mofidfar M, Zare RN, Gnanamani E. Cross-Coupling between Arylboronic Acids and Terminal Alkynes in Water Microdroplets. J Am Chem Soc. 2025;147(29):25779-25786. doi:10.1021/jacs.5c07509
You may like
Related articles And Qustion
Lastest Price from 4-Methoxyphenylboronic acid manufacturers
US $0.00/kg2025-03-03
- CAS:
- 5720-07-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS
US $34.00-1.20/kg2024-04-08
- CAS:
- 5720-09-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available