The solubility of Succinic anhydride
Introduction
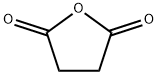
Succinic anhydride (C4H4O3, molecular weight 100.07, CAS No. 108-30-5) is a white crystal with wide use and optimum application prospects. Succinic anhydride is a cyclic dicarboxylic anhydride and a tetrahydrofuran dione. It is functionally related to a succinic acid. Succinic anhydride is a natural product found in Clerodendrum japonicum, Nicotiana tabacum, and other organisms with data available. It is manufactured by liquid-phase catalytic hydrogenation of maleic anhydride.
Uses
As an essential pharmaceutical intermediate and chemical material, succinic anhydride has been widely used in manufacturing drugs, resins, plastics, coatings, plasticizers, and coloring matter. For instance, succinic anhydride could be prepared with vitamin A and sulfonamides to prevent and treat bacterial infections. It is also used in the production of bactericides, pesticides, and plant growth regulators in agriculture.
Solubility

The IUPAC Name of Succinic anhydride is oxolane-2,5-dione. In the synthesis and purification process of this compound, the solubility of it in organic solvents directly affects the size of crystal formation, yield, and cost of production. It is soluble in chloroform, carbon tetrachloride, and alcohol and very slightly soluble in ether and water.
The solubility of succinic anhydride in acetone, acetonitrile, 2-propanol, ethyl acetate, acetonitrile + ethyl acetate, and acetone + ethyl acetate was determined with a temperature range from 278.15 to 333.15 K under atmospheric pressure using the analytical stirred-flask method[1]. The solubility of this organic compound in these solvents increased with increasing temperature. The solubility of succinic anhydride increases with temperature in different solvents, but the solubility rate is different. In different pure organic solvents: for temperatures between 278.15 to 298.15 K, the solubility of it decreases in the sequence acetonitrile, acetone, 2-propanol, and ethyl acetate; for temperatures between 298.15 to 333.15 K, the solubility of succinic anhydride in different solvents is in the following: acetone> acetonitrile> 2-propanol> ethyl acetate; among these solvents, the solubility of succinic anhydride is the lowest in ethyl acetate.
Succinic acid and succinic anhydride
Succinic acid and succinic anhydride occur frequently in nature. Their chemical behavior is characterized by the reactivity of the two carboxylic functions, i.e., hydration/dehydration, esterification, hydrogenation, acylation, and reactions with nitrogen and sulfur compounds, as well as of the two methylene groups, i.e., oxidation, halogenation, and condensation with aldehydes and ketones[2]. Succinic anhydride hydrolyzes readily to give succinic acid:
(CH2CO)2O + H2O → (CH2CO2H)
With alcohols (ROH), a similar reaction occurs, delivering the monoester:
(CH2CO)2O + ROH → RO2CCH2CH2CO2H.
References
[1] Wenge Yang . “Solubility of succinic anhydride in different pure solvents and binary solvent mixtures with the temperature range from 278.15 to 333.15 K.” Journal of Molecular Liquids 180 (2013): Pages 7-11.
[2] Fumagalli, C. “Succinic Acid and Succinic Anhydride.” 2006. 0.
You may like
Related articles And Qustion
See also
Lastest Price from Succinic anhydride manufacturers

US $0.00/kg2025-09-26
- CAS:
- 108-30-5
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 200tons

US $2.00/kg2025-08-15
- CAS:
- 108-30-5
- Min. Order:
- 1kg
- Purity:
- 99.9% min
- Supply Ability:
- 5000mt