Succinic anhydride: applications in diverse industries
General Description
Succinic anhydride is a compound that is commonly used in various applications. One such application is the modification of polysaccharides through esterification with octenyl succinic anhydride. This process results in the formation of octenyl succinic anhydride-polysaccharide derivatives, which exhibit amphiphilic properties. Another notable application of succinic anhydride is its use in the preparation of a highly effective biocatalyst. This biocatalyst is utilized to enhance the removal of bisphenol A (BPA) from wastewater, providing an efficient solution for environmental remediation. In the field of protein modification, succinic anhydride plays a crucial role. It selectively reacts with the free amino groups of lysine residues within proteins. This targeted reaction allows for specific protein modifications, influencing their structure and properties. To summarize, succinic anhydride finds applications in the esterification of polysaccharides, the preparation of robust biocatalysts for wastewater treatment, and the modification of proteins by reacting with free amino groups.

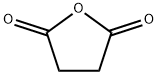
Figure 1. Succinic anhydride
Applications
Modification of polysaccharides
One such application is the esterification of polysaccharides with octenyl succinic anhydride, leading to the formation of octenyl succinic anhydride-polysaccharide derivatives with amphiphilic properties. These derivatives exhibit enhanced interfacial properties, improved emulsifying stability, increased viscosity, and reduced digestibility. In recent years, significant progress has been made in developing octenyl succinic anhydride-polysaccharides-based nano-encapsulation systems for hydrophobic bioactive compounds. These nano-encapsulation systems encompass diverse structures such as nanoemulsions, nanocapsules, nanoparticles, micelles, vesicles, and molecular inclusion complexes. Moreover, emphasis is placed on different nano-encapsulation systems utilizing octenyl succinic anhydride-polysaccharides as wall materials. Future perspectives in this field involve the development of octenyl succinic anhydride-polysaccharides-based nano-encapsulation systems with optimized functional properties. These systems aim to enhance the bioavailability and enable targeted delivery of various hydrophobic bioactive compounds. 1
Preparation of biocatalyst
Succinic anhydride has been applied in the preparation of a robust biocatalyst for enhancing the removal of bisphenol A (BPA) in wastewater. The process involves the reaction of succinic anhydride with laccase to obtain succinic anhydride-modified laccase (SA-laccase). Subsequently, SA-laccase is co-crystallized with Cu3(PO4)2 to form SA-laccase@Cu3(PO4)2 hybrid nanoflowers (hNFs). The activity of SA-laccase@Cu3(PO4)2 hNFs is significantly increased compared to other immobilized laccase systems such as bare laccase@Cu3(PO4)2, laccase@Ca3(PO4)2, and laccase@epoxy resin. The SA-laccase@Cu3(PO4)2 hNFs demonstrate 5.27 U/mg activity, which is 1.86-, 2.88-, and 2.15-fold higher than the aforementioned systems, respectively. Furthermore, the SA-laccase@Cu3(PO4)2 hNFs exhibit enhanced activity and stability under pH and high temperature conditions during BPA removal compared to free laccase. Optimum conditions for BPA removal using SA-laccase@Cu3(PO4)2 hNFs are observed at pH 6.0 and 35 °C, resulting in a BPA removal rate of 93.2%. This rate is 1.21-fold higher than that achieved using free laccase. Additionally, the SA-laccase@Cu3(PO4)2 hNFs retain approximately 90% of their initial catalytic activity for BPA removal after eight consecutive batch cycles. This method of preparing immobilized laccase can be further developed and improved to produce green biocatalysts for the efficient removal of persistent organic pollutants in wastewater. 2
Modification of proteins
In proteins, succinic anhydride is used for protein modification by reacting specifically with the free amino groups of lysine residues. This reaction replaces the amino group (NH3+) with an NHCOCH2CH2COO- functional group at neutral pH. A similar mechanism can be considered for alkenyl-substituted succinic anhydride. The reaction between succinic anhydride and the free amino groups of proteins results in N-acylation and introduces a charge into the protein's structure. This introduced charge is reported to have significant effects on the protein's structure and properties. The application of succinic anhydride for biopolymer modification was first mentioned in relation to soy protein isolate, where it was used as a paper coating agent. In aqueous medium, succinic anhydride reacts with proteins to form synthetic lipoproteins. The preferred sites for the nucleophilic substitution by succinic anhydride are the lysine residues. The reaction is pH-dependent and occurs rapidly at higher pH levels. At higher pH, non-ionized amino groups react with succinic anhydride. The reaction shows a lower than first-order dependence on protein concentration. The protein components of wool and silk fibers can react with succinic anhydride covalently. 3
Reference
1. Zhao L, Tong Q, Geng Z, et al. Recent advances of octenyl succinic anhydride modified polysaccharides as wall materials for nano-encapsulation of hydrophobic bioactive compounds. J Sci Food Agric, 2022, 102(14):6183-6192.
2. Yang H, He P, Yin Y, et al. Succinic anhydride-based chemical modification making laccase@Cu3(PO4)2 hybrid nanoflowers robust in removing bisphenol A in wastewater. Bioprocess Biosyst Eng, 2021, 44(10):2061-2073.
3. Shah NN, Soni N, Singhal RS. Modification of proteins and polysaccharides using dodecenyl succinic anhydride: Synthesis, properties and applications-A review. Int J Biol Macromol, 2018, 107(Pt B):2224-2233.
You may like
Related articles And Qustion
Lastest Price from Succinic anhydride manufacturers

US $0.00/kg2025-09-26
- CAS:
- 108-30-5
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 200tons

US $2.00/kg2025-08-15
- CAS:
- 108-30-5
- Min. Order:
- 1kg
- Purity:
- 99.9% min
- Supply Ability:
- 5000mt