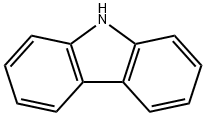
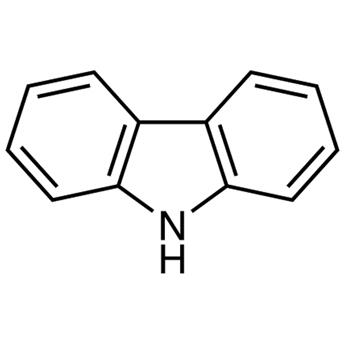
The physicochemical properties of carbazole
Introduction
Carbazole, also known as 9-nitrogen (hetero) fluorene (dibenzopyrrole), has a molecular formula of C12H9N and a molecular weight of 167.20. Carbazole is an important organic intermediate[1]. In addition to its traditional use in the synthesis of dyes, medicines and pesticides, carbazole and its derivatives are widely used in the synthesis of optoelectronic functional materials. On October 27, 2017, the list of carcinogens published by the World Health Organization's International Agency for Research on Cancer was preliminarily sorted for reference, and carbazole was included in the list of 2B carcinogens.

Picture 1 A bottom of carbazole
Physicochemical properties
Exposure to UV light shows strong fluorescence and prolonged phosphorescence. Easy sublimation. Very weak alkaline. Slightly soluble in ethanol, ether and benzene, soluble in quinoline, pyridine and acetone, slightly soluble in cold benzene, glacial acetic acid, chloroform, carbon disulfide and gasoline, soluble in concentrated sulfuric acid without decomposition, slightly soluble in petroleum ether, chlorinated hydrocarbons and acetic acid[2].
Preparation
In the laboratory method, the ring-closure dehydrogenation of o-aminobiphenyl is made from ammonia or chlorobenzene. In the industrial production method, 90% of the world's carbazole is obtained from coal tar; it can also be synthesized from o-aminobiphenyl, and then purified by recrystallization from xylene. (1) Synthesis method: 1-phenyl-1,2,3-benzotriazole is prepared by using o-aminodiphenylamine as raw material and treated with nitrous acid. After heating, nitrogen is lost to form carbazole. (2) Sulfuric acid method: Dissolve the crude anthracene with chlorobenzene or other solvents. The phenanthrene, fluorene and other substances in the crude anthracene are separated from anthracene and carbazole due to insolubility. Anthracene and carbazole are added to sulfuric acid for reaction. Then it forms carbazole sulfate with sulfuric acid and separates from anthracene. After hydrolyzing carbazole sulfate, the finished product is obtained by filtration and drying. (3) Solvent-rectification method: The crude anthracene is dissolved in the heavy benzene (160~200℃) by-product of coking, and the phenanthrene, fluorene and other substances in the crude anthracene are separated from anthracene and carbazole. High-temperature rectification is carried out in the distillation tower, and after one rectification, a product containing 85% to 90% of carbazole can be obtained, and the yield is 65%.
Application
Carbazole can be used to produce dyes, pigments, photoconductors, photosensitive materials, special inks, etc. The pigment produced with it is permanent purple RL, which is widely used in the coloring of automobile topcoat and high temperature resistant plastics, and has the advantages of high temperature resistance and ultraviolet light resistance. The dyestuffs sulphur vat blue RNX and Haichang blue produced with it have excellent fastness indicators, especially the fastness to chlorine bleaching. The blue varieties include carbazole IDM, carbazole LR, carbazole LB, and carbazole L3B. , the black varieties have carbazole black D. It also produces carbazole bisoxazine violet, a blue-violet pigment used in coatings, printing inks, carbon paper, and more. Carbazole is used in the production of sulfide reduced blue RNX, direct lightfast blue FFRL, FFGL, etc. It can also make leather, N-vinylcarbazole plastics, pesticides and insecticides tetranitrocarbazole, chlorinated carbazole, and UV-sensitive photographic dry films. In addition, carbazole has been increasingly used in the development of emerging optoelectronic new materials. The use of carbazole can prepare organic nonlinear optics (NLO) materials, organic electroluminescence (OEL) materials, photorefractive materials, containing Bifunctional system of carbazole chromophore, carbazole-containing photorefractive small molecular glass, etc.
Operation Disposal
Closed operation, local exhaust. Operators must undergo special training and strictly abide by operating procedures. It is recommended that the operator wear a dust mask (full face mask), a rubber protective clothing and rubber gloves. Keep away from fire and heat sources, and smoking is strictly prohibited in the workplace. Use explosion-proof ventilation systems and equipment. Avoid generating dust. Avoid contact with oxidants. When handling, it should be lightly loaded and unloaded to prevent damage to packaging and containers. Equipped with the corresponding variety and quantity of firefighting equipment and leakage emergency treatment equipment. Empty containers may be harmful residues.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. It should be stored separately from oxidants and edible chemicals, and should not be stored together. Use explosion-proof lighting and ventilation facilities. Prohibit the use of mechanical equipment and tools that are prone to sparks. Storage areas should be provided with suitable materials to contain spills.
Reference
1 Ye Cuiping, Zheng Huan, Wu Tingting, Fan Mingming, Feng Jie, Li Wenying, Study on Refining High Purity Carbazole from Anthracene Residue by Solvent Crystallization, [J], Coal Conversion, 2013.1, 36(1), 83-88
2 Tang Xinghua, Chen Kexin, Huang Xinyi, Li Xing, Application of carbazole compounds, [J], Materials Review A: Review, 2013.11, 27(11), 73-76
See also
Lastest Price from Carbazole manufacturers
US $1.00/KG2025-04-21
- CAS:
- 86-74-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/Kg/Bag2025-04-21
- CAS:
- 86-74-8
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 300mt/year