The carbazole drug
Introduction
Carbazoles are a significant class of nitrogen-containing heterocycles featuring a planar tricyclic skeleton that consists of two benzene rings (A rings) fused on either side of a central pyrrole ring (B ring). The carbazole structural motif is prevalent in, but not limited to, many naturally occurring alkaloids of plant or bacterial origin. Many of these alkaloids are medicinally helpful, exhibiting a rather impressive range of biological activities (anticancer, anti-HIV, antibacterial, anti-Alzheimer's, anticoagulant, analgetic, antiepileptic, antidiabetic, antioxidant, etc.)[1].
Structure
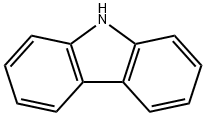
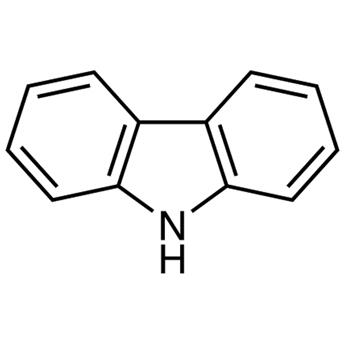
Carbazole is a polycyclic aromatic hydrocarbon consisting of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring, with a large, aromatic system and a central nitrogen atom showing extensive electron delocalization. The compound's structure is based on the indole structure, but a second benzene ring is fused onto the five-membered ring at the 2–3 position of indole. Carbazole is usually applied as a π-conjugated bridge to construct electron donor–π–π-electron acceptor (D–π–A) organic dyes. Organic dyes based on carbazole have shown important properties in dye-sensitized solar cells.
In medicine
Among the existing anticancer drugs, the carbazole scaffolds have been the key structural motif of many biologically active compounds for over half a century, including natural and synthetic products. Carbazole alkaloids originate in most cases from higher plants of the genera Murraya, Glycosmis, Clausena and Micromelum, all from the family of Rutaceae. Other sources are bacteria (e.g. Streptomyces), algae (e.g. Hyella caespitosa) and fungi (e.g. Aspergillus species)[2].
Carbazole is a "privileged scaffold" because it is a ligand for many receptors and possesses the only property to bind reversibly to enzymes. It provides a series of opportunities to study novel drugs that target one or more biological structures. The carbazole scaffold, commonly present in many biologically active pharmaceuticals and agrochemicals, is one of the most abundant heterocycles in nature. Several carbazole derivatives were individuated, and in recent years, an increase in interest in their use as bioactive molecules against different kinds of diseases has been recorded.
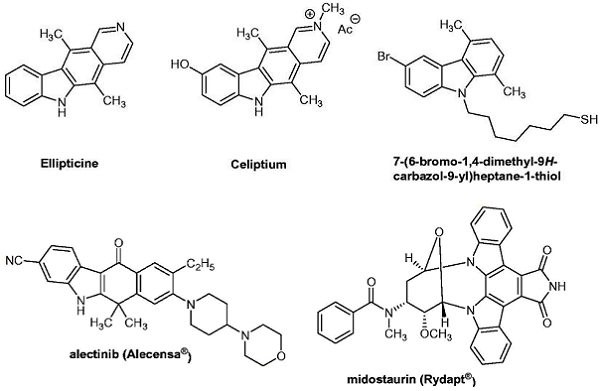
The parent compound 9H-carbazole was isolated from coal tar in 1872 by Graebe and Glazer. The first naturally occurring carbazole, the alkaloid murrayanine, was isolated from Murraya koenigii Spreng in 1962. Later, many carbazole derivatives have been synthesized and are well known for their pharmacological activities such as antioxidant, anti-inflammatory, antibacterial, anti-tumour, anti-convulsant, anti-psychotic and antidiabetic. Many carbazole derivatives and related compounds have been studied. More interestingly, three derivatives have obtained marketing authorization with anticancer drug status in different countries. Ellipticine, discovered in 1959 and extracted from the leaves of Ochrosia elliptica (Apocynacae) before being entirely synthesized, could be considered the first initial lead compound of carbazole analogues. After that, an ellipticine analogue named N-methyl-9-hydroxyellipticinium acetate (Celiptium®) was developed. Since 1982, Celiptium® has been used in the treatment of metastatic breast cancer. As reported by the National Cancer Institute (NCI) drug dictionary, N-methyl-9-hydroxyellipticinium acetate acted as a topoisomerase II inhibitor and an intercalating agent, stabilizing the cleavable complex of topoisomerase II and inducing DNA breakages, thereby inhibiting DNA replication and RNA and protein synthesis. New N-thioalkylcarbazole derivatives were synthesized and evaluated in comparison to ellipticine.
Among the bioactive carbazole-type derivatives, 7-(6-bromo-1,4-dimethyl-9H-carbazol-9-yl)-heptane-1-thiol also needs to be mentioned. The second derivative to obtain marketing authorization was alectinib bearing a 5H-benzo[b]carbazol-11(6H)-one scaffold (AF802, CH 5424802, RG7853, RO5424802, Alecensa®). Alectinib, an orally available drug, was first approved in 2015 by the US Food and Drug Administration (FDA) for Genentech and then by the European Medicines Agency (EMA) for Roche Pharmaceuticals, with an indication as monotherapy for the treatment of adult patients with anaplastic lymphoma kinase (ALK)-positive advanced non-small-cell lung cancer (NSCLC). The third derivative recently approved in 2017 by the FDA and the EMA is midostaurin (CGP41251, PKC412, Rydapt®) (Novartis), described mainly as the first fms-like tyrosine kinase 3 (FLT3) inhibitor for newly diagnosed acute myeloid leukemia (AML) and for advanced systemic mastocytosis (SM).
References
[1] S. Issa. “Carbazole scaffolds in cancer therapy: a review from 2012 to 2018.” Journal of Enzyme Inhibition and Medicinal Chemistry 34 1 (2019): 1321–1346.
[2] Anna Caruso. “Carbazole Derivatives as Antiviral Agents: An Overview.” Molecules 24 10 (2019).
You may like
Related articles And Qustion
Lastest Price from Carbazole manufacturers
US $1.00/KG2025-04-21
- CAS:
- 86-74-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/Kg/Bag2025-04-21
- CAS:
- 86-74-8
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 300mt/year