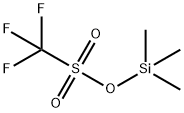
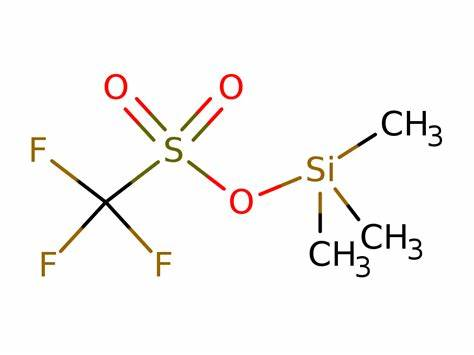
Trimethylsilyl Trifluoromethanesulfonate: A Versatile Reagent in Modern Organic Synthesis
Trimethylsilyl trifluoromethanesulfonate (TMSOTf) is a colorless moisture-sensitive liquid, it is frequent catalyzer or cationic initiator as silylation reagent in organic synthesis.
Properties
The trimethylsilyl trifluoromethanesulfonate (TMSOTf) promoted β-lactam fragmentation of 4-alkylthioazetidin-2-ones demonstrates a novel method to access N,S-acetals in moderate-to-excellent yields <1996JCS(P1)2321>. The reaction occurs by a nucleophilic attack of a nitrile group on the generated cation intermediate. 1
Applications
Trimethylsilyl Trifluoromethanesulfonate is mainly used to activate ketones and aldehydes in organic synthesis.
Preparation
The preparation method of Trimethylsilyl Trifluoromethanesulfonate:
in reaction vessel, add anhydrous trifluoromethanesulfonic acid and anhydrous trimethylchlorosilane; stirring reaction 12h under protection of inert gas; temperature of reaction is 10 ℃~30 ℃; after reaction finishes, underpressure distillation 1-2h, collects 125-135 ℃ of cut and is Trimethylsilyl Trifluoromethanesulfonate.
References:
[1] STARK H. Book Review: Comprehensive Organic Functional Group Transformations II by Alan Katritzky and Richard Taylor[J]. Archiv der Pharmazie, 2005, 338 11: 513-565. DOI:10.1002/ardp.200500196.Related articles And Qustion
See also
Lastest Price from Trimethylsilyl trifluoromethanesulfonate manufacturers

US $0.00/kg2025-09-22
- CAS:
- 27607-77-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 2500

US $0.00/KG2025-04-21
- CAS:
- 27607-77-8
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month