Trimethylsilyl trifluoromethanesulfonate: applications in organic synthesis
General Description
Trimethylsilyl trifluoromethanesulfonate (TMSOTf) is a trifluoromethanesulfonate compound with a trimethylsilyl R-group. It is commonly used in organic synthesis and has similar reactivity to trimethylsilyl chloride. TMSOTf acts as a catalyst and protective agent for aldehydes and ketones. It is involved in the conversion of ketones and aldehydes to silyl enol ethers, stereoselective synthesis of benzylated proanthocyanidin trimers, synthesis of naproxen-containing diaryliodonium salts, Koenigs-Knorr glycosylation reactions, and facilitation of the Friedel-Crafts addition reaction between indoles or N-alkylindoles and aldehydes.

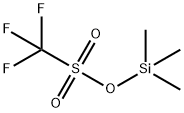
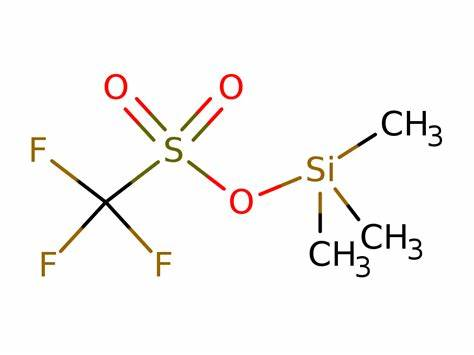
Figure 1. Trimethylsilyl trifluoromethanesulfonate
Applications in organic synthesis
The conversion of ketones and aldehydes to silyl enol ethers
The stereoselective synthesis of seven benzylated proanthocyanidin trimers (epicatechin-(4β-8)-epicatechin-(4β-8)-epicatechin trimer (procyanidin C1), catechin-(4α-8)-catechin-(4α-8)-catechin trimer(procyanidin C2), epicatechin-(4β-8)-epicatechin-(4β-8)-catechin trimer and epicatechin-(4β-8)-catechin-(4α-8)-epicatechin trimer derivatives) can be achieved with TMSOTf-catalyzed condensation reaction, in excellent yields. Deprotection of (+)-catechin and (-)-epicatechin trimer derivatives gives four natural procyanidin trimers in good yields. 1
Protection of aldehydes and ketones
TMSOTf exhibits a protective effect on aldehydes and ketones in organic transformations. In the presence of TMSOTf, a combination of PPh3 (triphenylphosphine) is used to selectively transform aldehydes into temporarily protected O,P-acetal type phosphonium salts. This protection allows for subsequent reactions involving other carbonyl groups in the substrate while keeping the aldehyde moiety intact. After these reactions, a hydrolytic work-up is performed, which liberates the aldehyde group, thus enabling a one-pot sequence comprising aldehyde protection, transformation of other carbonyl groups, and deprotection. Additionally, when PEt3 (triethylphosphine) is used instead of PPh3, ketones can be converted in situ to their corresponding O, P-ketal type phosphonium salts. This modification enables selective transformations of esters, Weinreb amides, and nitriles in the presence of ketones. 2
Synthesis of naproxen-containing diaryliodonium salts
TMSOTf plays a crucial role in the synthesis and functionalization of naproxen-containing diaryliodonium salts. In the process described, TMSOTf acts as an activator for the ArI(OH)OTs reagent, which is used in combination with naproxen methyl ester. The reaction takes place in a solvent mixture comprising dichloromethane and 2,2,2-trifluoroethanol (TFE). TMSOTf facilitates the activation of the ArI(OH)OTs reagent, enabling it to undergo reactions with the aromatic ring of the naproxen methyl ester. Furthermore, by subjecting the obtained 5-iodo-naproxen methyl ester to hydrolysis, 5-iodo-naproxen can be produced. This demonstrates the utility of TMSOTf in the synthesis and derivatization of naproxen derivatives. 3
Koenigs-Knorr Glycosylation Reaction
TMSOTf serves as a catalyst that significantly enhances the efficiency of traditional silver(I)-oxide-promoted glycosidations of glycosyl bromides, also known as the Koenigs-Knorr reaction. Typically, these glycosidation reactions require mild conditions to maintain a nearly neutral pH while achieving high reaction rates and excellent yields of glycosylation. The addition of catalytic TMSOTf facilitates these desirable reaction conditions. Moreover, the presence of TMSOTf unveils interesting reactivity trends among various differently protected glycosyl bromides. Specifically, benzoylated α-bromides exhibit greater reactivity compared to their benzylated counterparts under these reaction conditions. Overall, TMSOTf acts as a catalyst in this system, accelerating the glycosidation process, enabling mild reaction conditions, achieving neutral pH, and influencing the relative reactivity of different protected glycosyl bromides, particularly highlighting the enhanced reactivity of benzoylated α-bromides. 4
Facilitate the Friedel-Crafts addition reaction
TMSOTf is used as a reagent to facilitate the Friedel-Crafts addition reaction between indoles or N-alkylindoles and aldehydes. When TMSOTf is combined with a trialkylamine in the reaction mixture, it promotes the formation of 3-(1-silyloxyalkyl)indoles. This reaction involves the addition of an aldehyde to the indole or N-alkylindole substrate, resulting in the incorporation of a silyloxyalkyl group at the C-3 position of the indole ring. To ensure further reaction control, the reaction mixture is neutralized with pyridine. Following this, under basic conditions using tetrabutylammonium fluoride (TBAF), the protection of the intermediate product is removed, leading to the formation of the desired 1:1 adduct as a free alcohol. Importantly, this method prevents the undesired spontaneous conversion of the products to bisindolyl(aryl)methanes, which typically occurs when indoles are reacted with aldehydes under acidic conditions. By utilizing TMSOTf in combination with appropriate neutralization and deprotection steps, the formation of thermodynamically favored byproducts is minimized or eliminated. 5
Reference
1. Downey CW, Johnson MW, Tracy KJ. One-pot enol
silane formation-Mukaiyama aldol-type addition to dimethyl acetals
mediated by TMSOTf. J Org Chem, 2008, 73(8):3299-3302.
2. Yahata K, Minami M, Yoshikawa Y, Watanabe K, Fujioka H. Methodology for in situ protection of aldehydes and ketones using trimethylsilyl trifluoromethanesulfonate and phosphines: selective alkylation and reduction of ketones, esters, amides, and nitriles. Chem Pharm Bull, 2013, 61(12):1298-1307.
3. Zhou J, Bao Z, Wu P, Chen C. Preparation and Synthetic Application of Naproxen-Containing Diaryliodonium Salts. Molecules, 2021, 26(11):3240.
4. Singh Y, Demchenko AV. Koenigs-Knorr Glycosylation Reaction Catalyzed by Trimethylsilyl Trifluoromethanesulfonate. Chemistry, 2019, 25(6):1461-1465.
5. Downey CW, Poff CD, Nizinski AN. Friedel-Crafts Hydroxyalkylation of Indoles Mediated by Trimethylsilyl Trifluoromethanesulfonate. J Org Chem, 2015, 80(20):10364-10369.
Related articles And Qustion
Lastest Price from Trimethylsilyl trifluoromethanesulfonate manufacturers

US $0.00/kg2025-09-22
- CAS:
- 27607-77-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 2500

US $0.00/KG2025-04-21
- CAS:
- 27607-77-8
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month