Different properties of L-carnitine
Introduction
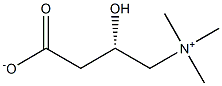
L-carnitine, also known as L-carnitine and vitamin BT, the chemical formula is C7H15NO3[1], the chemical name is (R) - 3-carboxyl-2-hydroxy-n, N, n-trimethylammonium propionate hydroxide internal salt, and the representative drug is L-carnitine. It is a kind of amino acid that promotes the conversion of fat into energy. The pure product is white crystal or white transparent fine powder [1]. It is very soluble in water, ethanol and methanol, slightly soluble in acetone, and insoluble in ether, benzene, chloroform and ethyl acetate. L-carnitine is easy to absorb moisture, has good water solubility and water absorption, and can withstand high temperatures above 200 ℃. It has no toxic and side effects on human body. Red meat is the main source of L-carnitine, which can also be synthesized by human body to meet physiological needs. It is not a real vitamin, but a substance similar to vitamins. It has many physiological functions such as fat oxidative decomposition, weight loss and anti fatigue. As a food additive, it is widely used in infant food, weight loss food, athlete food, nutritional supplements for the middle-aged and elderly, nutritional fortifiers for vegetarians and animal feed additives.

Picture 1 A bottle of L-carnitine health care products
Physical property
The appearance is white lens or white transparent fine powder, with a slight special fishy smell[2]. Very soluble in water, ethanol and methanol, slightly soluble in acetone, insoluble in ether, benzene, chloroform and ethyl acetate. It is easy to absorb moisture, and will deliquesce or even liquefy when exposed to air. It can be placed in the solution with pH value of 3 ~ 6 for more than 1 year, and can withstand the high temperature of more than 200 ℃. Its combined bond and binding group have good water solubility and water absorption. The specific rotation is - 30 ± 1 °.
Chemical property
L-carnitine belongs to amphoteric ion. When pH is neutral, L-carnitine exists in the form of lactone and has good stability. Unlike ordinary amino acids, L-carnitine does not carry an amine group, but a positively charged quaternary ammonium group, which is not affected by pH conditions. Moreover, the chelation process can not only form a four membered ring on its carboxyl group, but also form a six membered ring on hydroxyl and carboxyl groups. Therefore, it can form a variety of stable chelates with many metal ions, such as copper, zinc, manganese [2], magnesium, calcium, etc. the possible structure is shown in the figure below, In practical use, because L-carnitine has strong hygroscopicity and is easy to agglomerate at room temperature, it is usually prepared. Based on this principle, L-carnitine is prepared into non hygroscopic salts, such as calcium and magnesium salts of L-carnitine tartaric acid, L-carnitine fumarate and acetyl L-carnitine galactose. Now it has also been studied and synthesized into L-carnitine whey acid salt, which is convenient for storage, transportation and packaging.
Physiological function
L-carnitine widely exists in nature, and the content of carnitine in muscle tissue is high. Animal experiments showed that the concentration of L-carnitine in adrenal gland was the highest, followed by heart, bone, muscle, adipose tissue and liver. Free L-carnitine is excreted through urine [56]. Carnitine is required by the human body through dietary intake and / or endogenous synthesis. Human liver and kidney can synthesize carnitine from lysine and methionine, and need the assistance of VC, niacin, VB6 and iron. VC is necessary for carnitine biosynthesis. Among all nutritional cofactors, VC has the greatest effect on the rate of carnitine biosynthesis. Experiments have proved that the carnitine biosynthesis rate in the kidney of vitamin deficient guinea pigs is reduced by 8 ~ 10 times, and the heart and skeletal muscle are particularly sensitive to it. The concentration of carnitine in these tissues decreased by 50% in VC deficient animals. Iron is also needed in the response to VC. The concentration of carnitine in liver and heart tissue of pregnant rats with iron deficiency decreased significantly.
Reference
1 Goa K L, Brogden R N. L-carnitine[J]. Drugs, 1987, 34(1): 1-24.
2 Evans A M, Fornasini G. Pharmacokinetics of L-carnitine[J]. Clinical pharmacokinetics, 2003, 42(11): 941-967.
You may like
Related articles And Qustion
See also
Lastest Price from L-carnitine manufacturers

US $10.00/box2025-10-12
- CAS:
- 541-15-1
- Min. Order:
- 1box
- Purity:
- 99.99%
- Supply Ability:
- 100000box

US $0.00/Box2025-09-26
- CAS:
- 541-15-1
- Min. Order:
- 1Box
- Purity:
- 0.99
- Supply Ability:
- 10tons