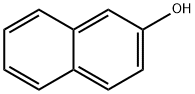
Different applications of 2-naphthol
Introduction
2-Naphthol (C10H8O) is a white to red flaky crystal that darkens upon long-term storage in air[1]. It is commonly used in the manufacture of torus acid, butyric acid, β-naphthol-3-carboxylic acid and azo dyes. It is also a raw material for rubber antioxidants, mineral dressing agents, fungicides, mildew inhibitors, preservatives, parasite control and anthelmintic drugs.

Picture 1 2-Naphthol flaky crystal
The toxicology of 2-Naphthol
2-Naphthol is toxicologically similar to phenol and is a stronger caustic. Has a strong irritating effect on the skin. Easily absorbed through the skin. Toxic effects on blood circulation and kidneys. In addition, it can also cause corneal damage. Although the lethal dose is not clear, there are cases of death caused by topical use of 3 to 4 g. The production equipment should be airtight and leak-proof, and it should be washed in time if it splashes on the skin. The workshop should be ventilated and the equipment should be airtight. Operators should wear protective equipment. 2-Naphthol is flammable, gradually darkens in color after prolonged storage, and is stable in air, but gradually darkens in color when exposed to sunlight. Heating sublimation, there is a pungent phenol odor. 2-Naphthol can be present in the flue gas. 2-Naphthol aqueous solution reacts with ferric chloride to show green color.
The preparation of 2-Naphthol
2-Naphthol can be obtained by sulfonation and alkali fusion of naphthalene. Sulfonated alkali fusion method is a widely used production method at home and abroad, but it has serious corrosion, high cost and high biological oxygen consumption in wastewater. The 2-isopropyl naphthalene method developed by American Cyanamid Company uses naphthalene and propylene as raw materials to produce 2-naphthol as a by-product of acetone. This method is similar to the cumene method for producing phenol. Raw material consumption quota: refined naphthalene 1170kg/t, sulfuric acid 1080kg/t, solid caustic soda 700kg/t.
The molten refined naphthalene was heated to 140°C, and 98% sulfuric acid was uniformly added within 20min in the ratio of naphthalene:sulfuric acid=1:1.085 (molar ratio), then the temperature was raised to 160~164°C for 2.5h sulfonation reaction , when the content of 2-naphthalene sulfonic acid reaches more than 66% and the total acidity is 25% to 27%, the reaction ends, and then the hydrolysis reaction is carried out at 160 ° C for 1 h, and the free naphthalene is blown off with steam at 140 ~ 150 ° C, and then slowly Evenly add sodium sulfite solution with a relative density of 1.14 preheated to 80-90°C for neutralization reaction until the Congo red test paper does not turn blue.
The sulphur dioxide gas generated in the reaction is removed with steam in time, the neutralized product is cooled to 35~40 ℃ for cooling and crystallization, the crystallization obtained by suction filtration is washed with 10% salt water, after suction dry, it is added to the molten 98 at 300~310 ℃. % sodium hydroxide, stir and maintain 320~330 ℃, make 2-naphthalene sulfonate sodium fuse into 2-naphthol sodium, then dilute the alkali fuse with hot water, and then feed into the sulfur dioxide generated by the above-mentioned neutralization reaction. , acidification reaction at 70 ~ 80 ℃ until phenolphthalein is colorless. The acidified product is allowed to stand for stratification, and the obtained upper layer liquid is heated to boiling, allowed to stand, and the water layer is separated.
The 1-naphthol in the 2-naphthol was removed by extractive crystallization. Mix 2-naphthol and water in a certain proportion and heat to 95°C. When 2-naphthol is melted, stir the mixture vigorously and rapidly cool down to about 85°C. Cool the crystalline slurry product to room temperature and filter. Purity analysis showed traces of 1-naphthol.
Anodic oxidation of 2-naphthol at boron-doped diamond electrodes
The anodic oxidation of 2-naphthol in acid media was investigated at a synthetic boron-doped diamond thin film electrode (BDD) using cyclic voltammetry and bulk electrolysis[1]. The results have shown that in the potential region, where the supporting electrolyte is stable, reactions involving simple electron transfer, such as oxidation of 2-naphthol to naphthoxy radical and 1,4-naphthoquinone occur. Polymeric materials, which lead to electrode fouling, are also formed in this potential region. Electrolysis at high positive potentials in the region of electrolyte decomposition causes complex oxidation reactions by electrogenerated hydroxyl radicals leading to the complete incineration of 2-naphthol. Electrode fouling is inhibited under these conditions. The experimental results have been also compared with a theoretical model. This model is based on the assumption that the rate of the anodic oxidation of 2-naphthol is a fast reaction and it is under diffusion control.
Recent advances in asymmetric oxidative coupling of 2-naphthol and its derivatives
The enantiomeric atropisomer of 1,1'-binaphthyl-2,2'-diol (BINOL) has become one of the most widely used chiral ligands and auxiliaries in asymmetric synthesis[2]. Optically active BINOLs can be selectively synthesized by direct asymmetric oxidative coupling of the same 2-naphthol and its substituted derivatives.
Electrochemical oxidation of 2-naphthol with in situ electrogenerated active chlorine
The electrochemical oxidation of 2-naphthol mediated by active chlorine electrogenerated in situ on a Ti–Ru–Sn ternary oxide has been studied by galvanostatic electrolysis under different experimental conditions[3]. Measurements of chemical oxygen demand (COD), HPLC and GC analyses have been used to follow the oxidation. In the absence of NaCl, only a small fraction of 2-naphthol was oxidized by direct electrolysis, while its complete mineralization was obtained in the case of chlorine-mediated electrolysis. In particular, the rate of naphthol oxidation was found to increase with chloride concentration, pH and to be independent of current density. Analysis of oxidation product has shown that initially organochlorinated compounds have been formed which have been further completely oxidized.
Photodegradation of 2-naphthol in water by artificial light illumination using TiO2 photocatalyst
The kinetics of the photocatalytic degradation of 2-naphthol has been investigated in aqueous suspensions of titanium dioxide (TiO2) under a variety of conditions, which is essential from application point of view[4]. The degradation was studied using different parameters such as types of TiO2, catalyst concentration, substrate concentration, reaction pH and in the presence of different electron acceptors such as hydrogen peroxide (H2O2), potassium bromate (KBrO3) and potassium persulphate (K2S2O8) besides molecular oxygen. The degradation rates were found to be strongly influenced by all the above parameters. The photocatalyst “Degussa P-25” was found to be more efficient as compared with other photocatalysts. The degradation kinetics fit well to the Langmuir–Hinshelwood rate law. It was found that an optimal concentration of 5 × 10−4 mol/l Ag+ in TiO2 achieved the fastest 2-naphthol degradation under the experimental conditions. However, with the addition of Na+, K+, Mg2+, Ca2+, Zn2+, Co2+ and Ni2+, there are no obvious effects on the reactions. An analysis of total organic carbon (TOC) showed that a complete mineralization of 2-naphthol can be easily achieved. The intermediate products were identified by HPLC–MS technique. A detailed degradation pathway could be proposed.
Reference
1 Laws W R, Brand L. Analysis of two-state excited-state reactions. The fluorescence decay of 2-naphthol[J]. Journal of Physical Chemistry, 1979, 83(7): 795-802.
2 Wang H. Recent advances in asymmetric oxidative coupling of 2‐naphthol and its derivatives[J]. Chirality, 2010, 22(9): 827-837.
3 Panizza M, Cerisola G. Electrochemical oxidation of 2-naphthol with in situ electrogenerated active chlorine[J]. Electrochimica acta, 2003, 48(11): 1515-1519.
4 Qourzal S, Barka N, Tamimi M, et al. Photodegradation of 2-naphthol in water by artificial light illumination using TiO2 photocatalyst: Identification of intermediates and the reaction pathway[J]. Applied Catalysis A: General, 2008, 334(1-2): 386-393.
Related articles And Qustion
See also
Lastest Price from 2-Naphthol manufacturers

US $0.00/kg2025-09-02
- CAS:
- 135-19-3
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $19.90/kg2025-04-21
- CAS:
- 135-19-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt