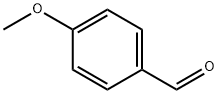
The introduction of p-Anisaldehyde
General description
p-Anisaldehyde is a natural product found in Vanilla pompona, Solidago odora, and other organisms with data available. The natural products occurrence database p-Anisaldehyde is a member of the class of benzaldehydes consisting of benzaldehyde itself carrying a methoxy substituent at position 4. It has a role as an insect repellent, a human urinary metabolite, a plant metabolite and a bacterial metabolite.
Application and Pharmacology
1.Condensation Product of p-Anisaldehyde(4-Methoxybenzaldehyde) and Ethylenediamine: Off-On Fluorescent Sensor for Cerium:he condensation product (L) of 4-methoxybenzaldehyde and ethylenediamine has been synthesised and characterised.L showed a 21 times enhancement in fluorescence intensity on interaction with Ce3+in CH3OH atλmax= 360 nm when excited with 270 nm photons. Metal ions K+, N a+, A l3+, C o2+, H g2+, C d2+, N i2+, Z n2+, M n2+, M g2+and Ca2+do not interfere. The stoichiometry of binding and the binding constants were determined from spectroscopic data and found to be 1:1 and 104.8M respectively. The detection limit was found to be 10–5.2M. The protonation/de-protonation of water molecules coordinated to Ce3+was found to show interesting behaviour on the fluorescence ofL: he condensation product of 4-methoxybenzaldehyde and ethylenediamine can detect Ce(III) ion over a host of other metal ions by fluorescentBoff-on mode in 1:1 (v/v) C H3OH:H2O .p-Anisaldehyde and CDCl3are from Sigma Aldrich, ethylenediamine and metal salts were either from Merck or Loba Chemie. The metal salts except Pb(NO3)2, C a C l2and HgCl2were sulphates. Metal salt solutions (0.01 M) were prepared in doubly distilled water obtained from quartz double distillation plant. The FT-IR spectra were recorded in a Perkin Elmer RXI spectrometer as KBr pellets,1H NMR and13C NMR spectra were recorded on a Bruker Ultra Shield 300 MHz spectrophotometer using CDCl3as solvent. The fluorescence and UV/Visible spectra were recorded in HITACHI 2500 and Shimadzu UV 1800 spectrophotometer respectively using quartz cuvette[1].
Figure1 Scheme 1Structure of the sensor
2.The antimicrobial activity and cellular pathways targeted by p-anisaldehyde and epigallocatechin gallate in the opportunistic human pathogen Pseudomonas aeruginosa. p-anisaldehyde from star anise into a polymer networkcalled PANDAS (Pro-Antimicrobial Networks via Degradable Acetals)and used it as a novel drug delivery platfor.PANDA sreleased p-anisaldehyde upon a change in pH and humidity and controlled growth of the multi-drug resistant pathogen Pseudomonas aeruginosa PAO1.In this study, we identified cellular pathways targeted by p-anisaldehyde by generating 10000 transposon mutants of PAo1 and screened them for hypersensitivity to p-anisaldehyde. To improve the antimicrobial efficacy ofp-anisaldehydewe combined it with epigallocatechin gallate(EGCG), a polyphenol from green tea,and demonstrated that it acts synergistically with p-anisaldehyde in killing P.aeruginosa. We then used RNA-seq to profile transcriptomic responses of P.aeruginosa to p-anisaldehyde, EGCG ,and their combination. The exposure to p-anisaldehyde altered the expression of genes involved in the modification of cell envelope membrane transport drug effluxeneray metabolism molybdenum cofactor biosynthesis and stress response. We also demonstrated that the addition of EGCG reversed many p-anisaldehyde coping effects and induce doxidative stress. Our results provide an insight into the antimicrobial activity of p-anisaldehyde and its interactions with EGCG and may aid in the rational[2].
Safety
The potential killing activity of p-anisaldehyde and its less cytotoxic effect encouraged us to study its mode of action. This work constitutes the first attempt to assess the antifungal role of p-anisaldehyde by studying its effect on sterol biosynthesis. Analysis of sterols obtained from all fluconazole-susceptible, and fluconazole-resistant, Candida isolates showed no major differences in either the sterol contents or the sterol patterns of the untreated controls of the isolates. Growth of Candida in the presence of sub-inhibitory and MIC90 concentrations of p-anisaldehyde altered the sterol patterns of both fluconazole-susceptible, and fluconazole-resistant, isolates. Studying the effect of this compound at various sub-inhibitory concentrations on the sterols of the tested Candida strains showed that this compound acts in a dose-dependent fashion to decrease ergosterol content. Near equal reduction of ergosterol in fluconazole-sensitive and -resistant strains at MIC―62% and 66% respectively―suggests that p-anisaldehyde acts by a more general mechanism. It lead to a decrease in NADPH concentration, which is vital for ergosterol biosynthesis. p-Anisaldehyde showed negligible toxicity as compared to fluconazole even at concentration values approaching MIC. In summary, p-anisaldehyde is found to be an effective antifungal against both fluconazole-resistant and sensitive strains of Candida. Ergosterol biosynthesis is strikingly reduced by p-anisaldehyde and its cause appears to be decreased NADPH routed through upregulation of aryl-alcohol dehydrogenases[3].
References
1.Adewunmi Y., Namjilsuren S. & Walker W. D. et al., "Antimicrobial Activity of, and Cellular Pathways Targeted by,p-Anisaldehyde and Epigallocatechin Gallate in the Opportunistic Human Pathogen Pseudomonas aeruginosa," Applied and Environmental Microbiology, Vol.86, No.4(2020).
2.Das D. K., Bharali B. & Goyari S., "Condensation Product of 4-Methoxybenzaldehyde and Ethylenediamine: “Off-On” Fluorescent Sensor for Cerium(III)," Journal of Fluorescence, Vol.28, No.6(2018), pp.1357-1361.
3.Shreaz S., Bhatia R. & Khan N. et al., "Exposure of Candida to p-anisaldehyde inhibits its growth and ergosterol biosynthesis," J Gen Appl Microbiol, Vol.57, No.3(2011), pp.129-136.
You may like
Related articles And Qustion
Lastest Price from p-Anisaldehyde manufacturers

US $15.00/KG2025-06-09
- CAS:
- 123-11-5
- Min. Order:
- 1KG
- Purity:
- 99
- Supply Ability:
- 1 ton

US $0.00/kg2025-04-21
- CAS:
- 123-11-5
- Min. Order:
- 1000kg
- Purity:
- 98.0%
- Supply Ability:
- 100ton/month