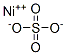The introduction of Nickel sulfate
General description
Anhydrous nickel sulfate is a yellow-green crystalline solid. Nickel sulfate can also be obtained as a hexahydrate which is blue to emerald green, and as a heptahydrate, which is green. Samples can contain variable quantities of water, depending on their previous exposure to moisture or conditions. All forms are mildly toxic and are carcinogenic. All are denser than water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Used to make other nickel compounds, in printing, and in dyeing of textiles. Nickel Sulfate is a yellow, green or blue colored, crystalline inorganic compound that produces toxic gases upon heating. Nickel sulfate is used in electroplating and as a chemical intermediate to produce other nickel compounds. Exposure to this substance can cause severe dermatitis, skin and asthma-like allergies and affects the lungs, kidneys, gastrointestinal tract and neurological system. Nickel sulfate is a known carcinogen and is associated with an increased risk of developing lung and nasal cancers. Nickel sulfate is a metal sulfate having nickel(2+) as the metal ion. It has a role as an allergen. It contains a nickel (2+).
Figure1 the chemical structure of nickel sulfate
Application and Pharmacology
1.Nickel sulfate induced apoptosis via activating ROS-dependent mitochondria and endoplasmic reticulum stress pathways in rat Leydig cells: Nickel can induce apoptosis of testicular Leydig cells in mice, whereas the mechanisms remain unclear. In this study, we investigated the role of nickel-induced reactive oxygen species (ROS) generation in mitochondria and endoplasmic reticulum stress (ERS) mediated apoptosis pathways in rat Leydig cells. Fluorescent DCF and Annexin-V FITC/PI staining were performed to measure the production of ROS and apoptosis in Leydig cells. RT-qPCR and Western blot were conducted to analyze the key genes and proteins involved in mitochondria and ERS apoptotic pathways. The results showed that nickel sulfate induced ROS generation, consequently resulted in nucleolus deformation and apoptosis in testicular Leydig cells, which were then attenuated by ROS inhibitors of N-acetylcysteine (NAC) and 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO). Nickel sulfate-triggered Leydig cells apoptosis via mitochondria and ERS pathways was characterized by the upregulated mRNA and proteins expression of Bak, cytochromec, caspase 9, caspase 3, GRP78, GADD153, and caspase 12, which were inhibited by NAC and TEMPO respectively. The findings indicated that nickel-induced ROS generation was involved in apoptosis via mitochondria and ERS pathways in rat Leydig cells[1].
2.Detoxification mechanisms of nickel sulfate in nematode Caenorhabditis elegans: the toxicity of nickel in wild-typeC. elegans. Growth, brood size, feeding behavior, and locomotion were tested in worms after being exposed to nickel sulfate with the aid of the high-throughput screening platform. The second aim was to apply the platform for investigating the effective detoxification mechanisms of nickel using specific transgenicC. elegans[2].
Synthesis
The Invention Relates to A Process for Preparing Nickel Sulfate Solution from Waste Nickel Metal Products. The waste nickel products can be prepared into nickel sulfate solution by means of crushing,sulfuric acid disso lution,resin impurity removal and other processes,which can turn waste into treasure. In this paper,the technological process of preparing nickel sulfate solution with this technology is introduced in detail,and the main environmental pollution problems,waste gas treatment measures and environmental risk prevention measures of this technology are analyzed. The preparation process of nickel sulfate takes waste nickel metal products and sulfuric acid as the main raw materials, and adopts treatment processes such as crushing, sulfuric acid dissolution and resin impurity removal. The product is nickel sulfate solution. This technology can realize the comprehensive utilization of industrial waste, and achieve the harmonious unity of economic benefits, social benefits and environmental benefits in the industrial model of circular economy[3].
Safety
Nickel is the most prevailing metal allergen with the highest sensitization rate among the“TOP 25” contact allergens and can affect about 15% of the human population. It is an essential trace metal in plants, animals, and humans. However, the environmental levels of nickel are considerably higher than what is needed for human life. Exposure to high levels of nickel can lead to skin allergies, lungfibrosis, and carcinogenesis. Few existing studies have closely examined the toxicity of nickel, let alone investigated the effective detoxification pathways.
Nickel can cause some changes in pituitary hormone secretion, and there is lymphocyte infiltration in the pituitary after higher dose exposure, indicating that the mechanism of subacute nickel exposure injury to the pituitary may be related to inflammatory reaction. The method of nickel exposure selected in this experiment is intraperitoneal injection. The dose of nickel exposure is small. Moreover, due to the existence of blood-brain barrier, subacute nickel exposure may not make a large amount of nickel deposit in the pituitary. The study found that the damage caused by nickel sulfate to cells will increase with the increase of nickel dose and the extension of action time. When it exceeds a certain dose, obvious cytotoxicity will appear[4].
References
1.Zou L., Su L. & Sun Y. et al., "Nickel sulfate induced apoptosis via activating ROS-dependent mitochondria and endoplasmic reticulum stress pathways in rat Leydig cells," Environmental Toxicology, Vol.32, No.7(2017), pp.1918-1926.
2.Tang B., Williams P. L. & Xue K. S. et al., "Detoxification mechanisms of nickel sulfate in nematode Caenorhabditis elegans," Chemosphere, Vol.260(2020), p.127627.
3.Zhang Dan, Wang Xinyu, Huang Tianlong: "a process for preparing nickel sulfate solution from waste nickel metal products", Gansu metallurgy, No. 01, 2022, pp. 100-102.4.Effect of nickel sulfate on histopathology of pituitary gland in rats.
You may like
Related articles And Qustion
Lastest Price from Nickel sulfate manufacturers

US $1.00/kg2025-03-07
- CAS:
- 7786-81-4
- Min. Order:
- 1kg
- Purity:
- Ni:33%
- Supply Ability:
- 20tons

US $5.00/KG2023-03-27
- CAS:
- 7786-81-4
- Min. Order:
- 1KG
- Purity:
- 0.99
- Supply Ability:
- 5t