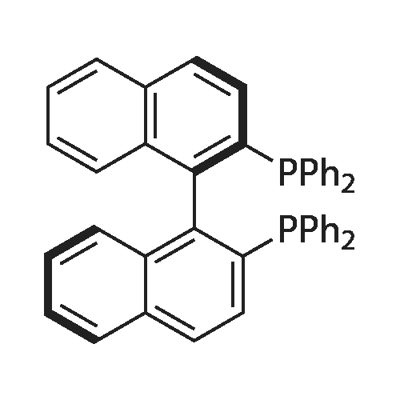
The applications and synthesize of 2-2-bis-diphenylphosphino-1-1-binaphthyl
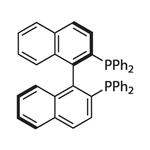
BINAP (2,2'-bis(diphenylphosphino)-1,1'-binaphthyl) is an organophosphorus compound. This chiral ligand is widely used in asymmetric synthesis. It consists of a pair of 2-diphenylphosphinonaphthyl groups linked at the 1 and 1´ positions. This C2-symmetric framework lacks a stereogenic atom, but has axial chirality due to restricted rotation (atropisomerism). The barrier to racemization is high due to steric hindrance, which limits rotation about the bond linking the naphthyl rings. The dihedral angle is approximately 90˚.
The following example is about its application on the synthesis of benzoxazine derivatives, where BINAP is a chiral ligand [1]
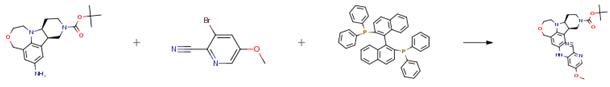
The title compound was prepared using tert-butyl (7bR,11aS)-6-amino-1,2,7b,10, 11,11a-hexahydro-4H-[1,4]oxazepino[6,5,4-hi]pyrido[4,3-b]indole-9(8H)-carboxylate from Example 56, Part B (130 mg, 0.377 mmol), 3-bromo-2-cyano-5- methoxypyridine (67 mg, 0.34 mmol), CsCO3 (256 mg, 0.787 mmol) in anhydrous toluene (10 mL), tris(dibenzylideneacetone)dipalladium (3.4 mg, 3.7 μmol), and 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl (7.0 mg, 11 μmol) to provide the corresponding tert-butyl (7bR,11aS)-6-(2-cyano-5-methoxy-3-pyridinyl)-amino -1,2,7b,10,11,11a-hexahydro-4H-[1,4]oxazepino[6,5,4-hi]pyrido[4,3-b]indole-9(8H)-carboxylate (146 mg) in 90 percent yield.
The following example is about its application on the synthesis of diazabicyclic agents, where BINAP is a chiral catalyst. [3]
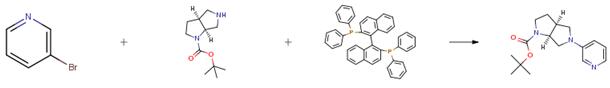
The starting material (0.71 g, 3.30 mmol) in toluene (33 mL) was treated with tris(dibenzylideneacetone)dipalladium(0) (61 mg, 0.10 mmol), 2,2'-bis(diphenyl phosphino)-1,1'-binaphthyl (BINAP) (83 mg, 0.10 mmol), 3-bromopyridine (0.58 g, 3.70 mmol), and sodium tert-butoxide (0.54 g, 5.60 mmol). After heating at 80° C. for 16 hours, the mixture was poured into diethyl ether (100 mL), washed with brine (100 mL), dried (MgSO4) and concentrated under reduced pressure. The residue was purified by chromatography (SiO2, 5 percent MeOH/CH2Cl2) to provide the product as a yellow oil (0.87 g, 91percent yield).
The following example is about its application on the synthesis of substituted azepino[4,5b] indoline derivatives, where BINAP is a chiral ligand [4]
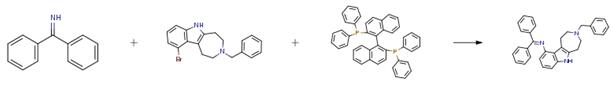
A mixture of 3-benzyl-10-bromo-1,2,3,4,5,6-hexahydroazepino[4,5-b]indole (0.71 g, 2.00 mmol), benzophenone imine (0.44 g, 0.40 mL, 2.40 mmol), tris(dibenzyl ideneacetone)dipalladium(0) (0.037 g, 0.040 mmol), (S)-(-)-2,2'-bis(diphenyl phosphino)-1,1'-binaphthyl (0.075 g, 0.120 mmol), and sodium tert-butoxide (0.269 g, 2.80 mmol) in toluene (20.0 mL) was refluxed for 16 h. After cooling to room temperature, water and ethyl acetate were added, and the phases separated. The aqueous layer was extracted with ethyl acetate. The combined ethyl acetate solution was dried (MgSO4) and filtered. The filtrate was concentrated in vacuo to dryness and the residue was subjected to column chromatography (silica gel, 30 percent EtOAc/hexane, 1 percent Et3N) to afford a yellow fluffy solid as the product (0.855 g, 94 percent): mp > 71° C.
References
1. Roche Palo Alto LLC. Benzoxazine derivatives and uses thereof US2003/232825[P], 2003, A1, Page 31.
2. Robichaud AJ, Fevig JM, Mitchell IS, Lee T, Chen W, Cacciola J. Substituted pyridoindoles as serotonin agonists and antagonists. US2004/186094[P], 2004, A1.
3. Schrimpf MR, Tietje KR, Toupence RB, Ji J, Basha A, Bunnelle WH, Daanen JF, Pace JM, Sippy KB. Diazabicyclic central nervous system active agents. US2002/19388[P], 2002, A1.
4. Frank KE, Acker BA, Ennis MD, Fisher JF, Fu J, Jacobsen EJ, McWhorter JR, William W, Morris JK, Rogier DJ. Substituted azepino[4,5b] indoline derivatives. US2002/77318[P], 2002, A1
You may like
Related articles And Qustion
See also
Lastest Price from (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 76189-55-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt
US $0.00/Kg2025-04-14
- CAS:
- 76189-55-4
- Min. Order:
- 1Kg
- Purity:
- 98%
- Supply Ability:
- 100kg