Role of voglibose in prevention of type 2 diabetes
Description
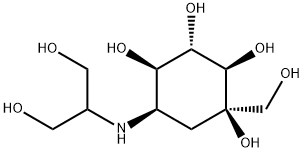
Voglibose (INN and USAN, trade name Voglib, marketed by Mascot Health Series) is an alpha-glucosidase inhibitor used for lowering post-prandial blood glucose levels in people with diabetes mellitus. Voglibose delays the absorption of glucose thereby reducing the risk of macrovascular complications. Voglibose is a research product of Takeda Pharmaceutical Company, Japan's largest pharmaceutical company. Voglibose was first launched in 1994, under the trade name BASEN, to improve postprandial hyperglycemia in diabetes mellitus.
Background
The increased prevalence of type 2 diabetes mellitus is a major concern for the health providers. We have done an observation study in the diagnosed IGT patient who received α-glucosidase inhibitor (voglibose), which could prevent the development of type 2 diabetes in high-risk individuals.
Methods
This study was an observational study comprising of voglibose and placebo in individuals with impaired glucose tolerance.66 eligible patients were on the standard diet and taking regular exercise with impaired glucose tolerance were randomly assigned to oral voglibose 0.2 mg three times a day (n=66) or placebo (n=60) in this study. Treatment was continued until participants developed type 2 diabetes (primary endpoint) or normoglycaemia (secondary endpoint).
In the final analysis, 66 registered individuals fulfilled the inclusion criteria: 36 were randomly assigned to receive voglibose and 30 placebos (two participants in the placebo group did not take their medication and were excluded).
Results
The mean duration of treatment was 48.3 weeks (SD: 36.4), i.e., 45.4 weeks (34.7) for voglibose and 51.7 weeks (37.4) for placebo. Voglibose was better than placebo (p= 0.0024) in individuals treated for an average of 48.3 weeks (SD 36.4). Patients treated with voglibose had a lower risk of progression to type 2 diabetes than did those on placebo. More people in the voglibose group achieved normoglycaemia than did those in the placebo group.
Conclusion
Voglibose, in addition to lifestyle modification, can reduce the development of type 2 diabetes in high risk individuals with impaired glucose tolerance.Postprandial hyperglycemia (PPHG) is primarily due to first phase insulin secretion. Alpha glucosidase inhibitors delay glucose absorption at the intestine level and thereby prevent sudden surge of glucose after a meal.
There are three drugs which belong to this class, acarbose, miglitol and voglibose, of which voglibose is the newest.
Related articles And Qustion
See also
Lastest Price from Voglibose manufacturers

US $0.00-0.00/g2025-05-21
- CAS:
- 83480-29-9
- Min. Order:
- 1g
- Purity:
- 99%min
- Supply Ability:
- 1000 G

US $1.00/kg2025-04-21
- CAS:
- 83480-29-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt