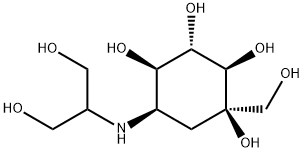
Voglibose: mechanism of action, pharmacokinetics and safety
General Description
Voglibose is an α-glucosidase inhibitor used as an adjunctive treatment for type 2 diabetes mellitus. Its mechanism of action involves inhibiting enzymes called α-glucosidases in the small intestine, leading to delayed carbohydrate digestion and absorption. This helps regulate postprandial blood glucose levels without causing hypoglycemia. Voglibose is poorly absorbed orally and primarily acts within the gastrointestinal tract. It is metabolized by intestinal enzymes and microbial flora and is predominantly excreted in stools. The drug has a generally favorable safety profile, although it may cause gastrointestinal side effects such as flatulence, abdominal distension, diarrhea, and abnormal bowel sounds. These side effects usually improve over time and can be managed by gradually increasing the dosage and adjusting the diet. When used in combination with other antidiabetic medications, voglibose did not show significant adverse effects.
Figure 1. Tablets of voglibose
Mechanism of action
Voglibose is a medication that exerts its effect by inhibiting a group of enzymes called α-glucosidases. These enzymes are present in the brush border of enterocytes, which are cells lining the small intestine. α-glucosidases are responsible for breaking down nonabsorbable oligosaccharides and polysaccharides into smaller, absorbable monosaccharides, such as glucose. By competitively inhibiting the action of α-glucosidases, voglibose slows down the digestion and absorption of carbohydrates in the digestive system. This delay in carbohydrate breakdown leads to a gradual release of glucose into the bloodstream, preventing a rapid increase in postprandial (after-meal) plasma glucose levels. Additionally, since voglibose does not stimulate insulin secretion, it does not cause hypoglycemia (low blood sugar levels). Overall, voglibose helps regulate blood glucose levels by reducing the spike in postprandial glucose and insulin secretion after meals. It is commonly used as an adjunctive treatment for type 2 diabetes mellitus to improve glycemic control. 1
Pharmacokinetics
Voglibose exhibits unique pharmacokinetic characteristics. When taken orally, it is slowly and poorly absorbed, leading to undetectable plasma concentrations at therapeutic doses. The majority of the active unchanged drug remains in the gastrointestinal tract, where it undergoes metabolism by intestinal enzymes and microbial flora. Notably, no active metabolites have been identified thus far. In terms of elimination, voglibose is rapidly excreted in stools, while renal excretion is insignificant. These elimination pathways suggest that the drug's primary route is through fecal excretion. Regarding drug interactions, voglibose has been found to have no significant impact on the pharmacokinetics of several other medications, including warfarin, hydrochlorothiazide, digoxin, glibenclamide, dapagliflozin, and vildagliptin. This indicates that concomitant administration of voglibose with these drugs does not typically result in alterations in their absorption, distribution, metabolism, or excretion. Overall, voglibose's pharmacokinetic features include poor oral absorption, predominant activity within the gastrointestinal tract, rapid excretion in stools, negligible renal excretion, and minimal impact on the pharmacokinetics of co-administered drugs. 2
Safety
Voglibose has a generally favorable safety profile with some gastrointestinal side effects. Due to its mechanism of action, complex carbohydrates are not fully degraded to glucose, leading to increased carbohydrate delivery to the colon for digestion by microbial flora. This can result in gastrointestinal symptoms, such as flatulence, abdominal distension, diarrhea, and abnormal bowel sounds. However, these side effects usually diminish with continued treatment and can be minimized by gradually increasing the dosage and selecting an appropriate diet. In a large study involving Japanese individuals with impaired glucose tolerance (IGT), the incidence of possible treatment-related adverse events was higher with voglibose compared to placebo. Common side effects included flatulence, abdominal distension, diarrhea, and abnormal bowel sounds, but they were considered mild-to-moderate in severity. The discontinuation rate due to adverse events did not significantly differ between voglibose and placebo. When combined with other antidiabetic medications like pioglitazone or mitiglinide in patients with type 2 diabetes on hemodialysis, voglibose did not cause serious adverse effects such as hypoglycemia or liver impairment. 3
Reference
1. Kaku K. Efficacy of voglibose in type 2 diabetes. Expert Opin Pharmacother, 2014, 15(8):1181-1190.
2. Derosa G, Maffioli P. Alpha-glucosidase inhibitors and their use in clinical practice. Arch Med Sci, 2012, 8(5):899-906
3. Intestinal alpha-glucosidase inhibitors: abdominal gas cysts. Prescrire Int, 2012, 21(130):212-213.
You may like
Related articles And Qustion
Lastest Price from Voglibose manufacturers

US $0.00-0.00/g2025-05-21
- CAS:
- 83480-29-9
- Min. Order:
- 1g
- Purity:
- 99%min
- Supply Ability:
- 1000 G

US $1.00/kg2025-04-21
- CAS:
- 83480-29-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt