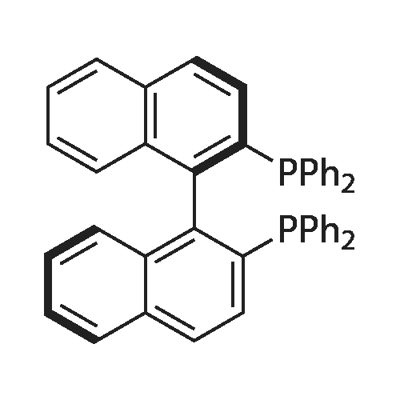
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl: properties and applications in organic synthesis
General Description
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl, or (R)-BINAP, is a highly valuable chiral diphosphine ligand widely utilized in asymmetric catalysis. Its exceptional properties, including high stereoselectivity, formation of stable metal complexes, and modular structure for structural modifications, make it indispensable in organic synthesis. In particular, (R)-BINAP is crucial in promoting highly enantioselective carbonitration reactions in the presence of palladium catalysts, resulting in the production of alkyl nitrates with high enantiomeric excess. Additionally, it plays a pivotal role in homochiral-type ligation, influencing the assembly of chiral ligands onto metal clusters and contributing to the development of enantioselective reactions. Overall, (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl's significance in asymmetric catalysis is evident in its diverse applications, making it an indispensable tool for the advancement of chemical synthesis.

Figure 1. (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
Properties
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl is a chiral diphosphine ligand widely used in asymmetric catalysis. This compound is known for its exceptional properties that make it a valuable tool in organic synthesis. Firstly, (R)-BINAP exhibits high stereoselectivity due to its chirality. As a result, it is particularly effective in promoting asymmetric transformations, enabling the formation of enantiomerically pure products. This property makes (R)-BINAP highly desirable in the production of pharmaceuticals and other fine chemicals where chirality is critical. Secondly, (R)-BINAP forms stable complexes with various metal catalysts, such as palladium, rhodium, and nickel. These complexes exhibit excellent reactivity and selectivity in a wide range of catalytic processes including hydrogenation, hydroformylation, and C-C bond forming reactions. Moreover, (R)-BINAP's modular structure allows for structural modifications, leading to derivatives with tailored properties for specific applications. Its versatility and reliability have established (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl as a cornerstone in the field of asymmetric catalysis, contributing significantly to the advancement of diverse chemical synthesis. 1
Applications in organic synthesis
Carbonitration reactions
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl is a chiral ligand widely employed in asymmetric catalysis. In the context of the provided text, (R)-BINAP is utilized in the Pd0-catalyzed asymmetric carbonitration of (Z)-1-iodo-1,6-dienes to produce alkyl nitrates with high enantiomeric excess (ee) up to 96%. The reaction takes place under a toluene/H2O biphasic system in the presence of an H-bonding donor [PyH][BF4] and AgNO3 additive. The key to this process lies in the combination of (R)-BINAP as the chiral ligand, which imparts stereochemical control, along with the H-bonding interaction of PyH⋅⋅⋅ONO2, which facilitates the dissociation of the O2NO- ligand from the alkyl-PdII-ONO2 species. This enables the challenging alkyl-PdII-ONO2 reductive elimination—a crucial step in the formation of alkyl nitrates—to become feasible. Mechanistic studies have further revealed that the reaction proceeds through oxidative addition, anion ligand exchange, alkene insertion, and SN2-type alkyl-PdII-ONO2 reductive elimination. In conclusion, (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl plays a pivotal role in enabling highly enantioselective carbonitration reactions, showcasing its importance in asymmetric catalysis. 2
Homochiral-type ligation
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl plays a crucial role in the preference of homochiral-type ligation. Specifically, the article discusses the assembly of SS-type ligands onto Au11 clusters protected by diphosphine S,S-DIOP. The synthesized and isolated Au11 clusters, designated as Au11(S,S-DIOP)4(rac-/R-/S-BINAP), exhibit optical and chiroptical responses that are characterized based on the handedness of BINAP. Notably, the absorption spectra of these Au11 clusters are nearly identical, but their circular dichroism (CD) profiles vary depending on the handedness of (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl. The article further reports a small yet distinctive preference for (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl ligation, leading to homochiral-type (SS-type) ligand assembly formation. Quantum chemical calculations and experimental observations indicate this preference, which may be attributed to the overall ligand structures and assemblies involving interligand interactions. This finding is significant for the development of enantioselective reactions, as it sheds light on chiral sorting and amplification processes through the control of homochirality or heterochirality. It is anticipated that the understanding gained from this research will contribute to further advancements in enantioselective reactions based on various metal clusters with chiral ligands. 3
Reference
1. Bunten KA, Farrar DH, Lough AJ. (R)-(+)-2,2'-Bis(diphenylphosphinoyl)-1,1'-binaphthyl. Acta Crystallogr C. 2000 Jun 1;56(Pt 6):E267.
2. Qi L, Dong M, Qian J, Yu S, Tong X. Pd0 -Catalyzed Asymmetric Carbonitratation Reaction Featuring an H-Bonding-Driven Alkyl-PdII -ONO2 Reductive Elimination. Angew Chem Int Ed Engl. 2023 Jan 16;62(3):e202215397.
3. Sato Y, Yao H. Mixed-diphosphine-protected chiral undecagold clusters Au11(S,S-DIOP)4(rac-/R-/S-BINAP): effect of the handedness of BINAP on their chiroptical responses. Phys Chem Chem Phys. 2021 Aug 12;23(31):16847-16854.
Related articles And Qustion
Lastest Price from (R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 76189-55-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt
US $0.00/Kg2025-04-14
- CAS:
- 76189-55-4
- Min. Order:
- 1Kg
- Purity:
- 98%
- Supply Ability:
- 100kg