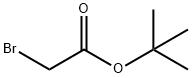
tert-Butyl bromoacetate: Background technique, synthesis, applications and hazard
General description
Tert-Butyl bromoacetate is a light yellow transparent liquid, soluble in alcohols, ethers and other organic solvents for the synthesis of amino acids and peptides, and the synthesis of cyclic polyamino carboxylate contrast agents, cephalosporins, Receptor antagonists and other drugs important intermediates, or common alkylating agent. Tert-Butyl bromoacetate has fewer domestic producers, especially those with larger output, which makes the country must rely on imports to meet the demand, which largely restricts the development of tert-butyl bromoacetate in China [1]. Its appearance is as follows:

Figure 1 Appearance of tert-Butyl bromoacetate
Background technique
Tert-butyl bromoacetate is an important pharmaceutical intermediate. Currently, the main production method used at home and abroad is described in the document "Convenient Synthesis of Tert-Butyl Bromoacetate". This method is a two-step method. The main process is described as follows:
Bromoacetic acid + sulfoxide chloride → bromoacetyl chloride
Bromoacetyl chloride + tert-butanol → tert-butyl bromoacetate + hydrogen chloride
In the data of the reaction laboratory, the excess of sulfoxide chloride and the yield of bromoacetyl chloride are 95%. The process must use atmospheric distillation and vacuum distillation successively. The bromoacetyl chloride obtained by the reaction, then bromoacetyl chloride and tertiary Butanol reaction, with sodium tripolyphosphate as acid binding agent, and adding chloroform to carry out the reaction, the reaction product was washed with inorganic salts, dried, then atmospheric distillation and vacuum distillation, and finally obtained tert-butyl bromoacetate, the first. The two-step yield is 93%, and the total yield of the two-step laboratory is 89%. The overall process is complicated, and a large number of three wastes are generated, which pollutes the environment and has high production costs. It is not suitable for scale and continuous production [2].
Synthesis
N,N-Dimethylaniline (160 mL, 153 g, 1.27 mol, 1.1 eq.) and dry t-butanol (144 mL, 111 g, 1.50 mol, 1.3 eq.) were dissolved in absolute THF (200 mL) and bromoacetyl bromide (100 mL, 232 g, 1.15 mol) was added dropwise at 0 degC. The mixture was stirred overnight at room temperature, a white precipitate was formed, which dissolved during the subsequent addition of water (200 mL). The aqueous phase was separated, extracted twice with Et2O and the combined organic phases were then washed with 10 % H2SO4 until the neutralization of the aqueous phase with NaOH did not develop turbidity (about 400 mL). The organic phase was then still washed once each with water and sat. NaCl solution, dried over MgSO4 and the solvent removed under vacuum. The resulting crude product was purified by fractional distillation under vacuum and yielded 166 g (74 %) of tert-Butyl bromoacetate as a colorless liquid. Rf = 0.78 (CH2Cl2/ethyl acetate = 20:1). bp 66-68 degC (20 mbar). IR (neat) ν (cm-1): 1740. 1H-NMR (CDCl3) δ (ppm): 1.49 (s, 9H), 3.75 (s, 2H) [3].
Applications
Tert-butyl bromoacetate is a common alkylating agent used as a pharmaceutical intermediate. It is used for synthesizing amino acids and peptide compounds. Moreover, it is used for synthesizing collagenase inhibitors (S, S, R) - (-) actinin. For example, tert-butyl bromoacetate can be used for the synthesis of succinamide peptidomimetic inhibitors of cathepsin S as a alkylating agent, and preparation of isoquinoline derivatives as inhibitors of p38 mitogen-activated protein (p38MAP).
Hazard
Tert-butyl bromoacetate gives water an unpleasant taste at very low concentrations. There are currently no known published cases of any in-situ treatment method that has been capable of reducing contaminant concentrations to baseline (pre-development) conditions within the aquifer soil matrix. The United States Environmental Protection Agency (EPA) has concluded that available data are inadequate to quantify health risks of Tert-butyl bromoacetate at low exposure levels in drinking water, but the data support the conclusion that Tert-butyl bromoacetate is a potential human carcinogen at high doses. In addition, the tert-butyl bromoacetate has lachrymatory effect.
References
[1]Zhou et al. Process for environment-friendly synthesis of tert-butyl bromoacetate. China, CN106380398 A 2017-02-08.
[2]Liu et al. Process for synthesizing tert-butyl bromoacetate. China, CN102659588 A 2012-09-12.
[3]Fecher et al. Development of Highly Selective Glycosidic Pro-Drugs of CC-1065 Analogs and Estrone-Talaromycin Hybride Natural Products for Targeted Tumor Therapy. 2000, (20140701), No pp.
You may like
Related articles And Qustion
See also
Lastest Price from tert-Butyl bromoacetate manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 5292-43-3
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons

US $0.00-0.00/kg2025-04-21
- CAS:
- 5292-43-3
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons