Tert-Butyl bromoacetate: applications as alkylating agent and safety
General Description
Tert-butyl bromoacetate is employed as an alkylating agent in various synthetic processes, including the synthesis of 3-oxa-OPC homologues, C-ODTA, and nucleoside analogs. Its use facilitates the introduction of specific functional groups, enabling the desired structural transformations and enhancing the properties of the resulting compounds for applications in radiolabeling, bioconjugation chemistry, and pharmaceutical synthesis. However, it is important to note that tert-butyl bromoacetate poses significant health hazards, including flammability and potential skin, eye, and respiratory irritation. Therefore, careful handling and adherence to safety protocols are essential when working with this substance.

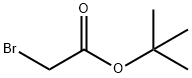
Figure 1. Tert-Butyl bromoacetate
Applications as alkylating agent
Synthesis of 3-oxa-OPC homologues
Tert-butyl bromoacetate is utilized in the synthesis of 3-oxa-OPC homologues through etherification of an alcohol under phase-transfer conditions. Etherification is a chemical reaction in which two reactants form an ether as the product. In this case, tert-butyl bromoacetate is used as the alkylating agent to introduce the tert-butyl group into the alcohol, resulting in the formation of the desired 3-oxa-OPC homologues. The use of tert-butyl bromoacetate in this context is crucial for introducing the necessary functional group and achieving the desired structural transformation. By employing phase-transfer conditions, which involve the transfer of a reactant from one phase to another, the synthesis can be carried out efficiently, allowing for the successful production of the targeted 3-oxa-OPC homologues. Overall, tert-butyl bromoacetate plays a pivotal role in the synthesis of 3-oxa-OPC homologues by serving as the alkylating agent in the etherification process under phase-transfer conditions, enabling the efficient and controlled introduction of the tert-butyl group to produce the desired compounds. 1
Synthetic of C-ODTA
Tert-Butyl bromoacetate is utilized in the synthetic procedure for C-ODTA as an alkylating agent. In the described approach, after the intramolecular cyclization reaction and reduction with BH(3), the resulting cyclic compound is alkylated with tert-butyl bromoacetate. This step introduces the tert-butyl group onto the macrocycle, leading to the formation of the desired C-ODTA. The use of tert-butyl bromoacetate in this context allows for the functionalization of the polyazamacrocycle, enabling the modification of its chelating properties and enhancing its performance as a chelating agent for radiolabeling peptides and proteins with (111)In. Overall, tert-butyl bromoacetate plays a crucial role in the synthetic pathway for C-ODTA by facilitating the introduction of specific functional groups onto the macrocycle, thereby contributing to its utility as a versatile chelating agent for various applications in radiolabeling and bioconjugation chemistry. 2
Synthesis of nucleoside analogs
Tert-Butyl bromoacetate, a versatile reagent with a bulky tert-butyl group and a reactive bromo functionality, plays a crucial role in the regioselective purine glycosylation and alkylation reactions mediated by the novel 2,3-dicyclohexylsuccinimide (Cy2SI) protecting group. In the context of purine chemistry, Tert-Butyl bromoacetate serves as an efficient alkylating agent due to its ability to introduce the tert-butyl ester functionality under mild reaction conditions. The bulky nature of the tert-butyl group promotes high regioselectivity during the alkylation of purines, ensuring the desired substitution occurs at the targeted position. Furthermore, the bromo functionality facilitates the formation of stable intermediates, enabling selective and efficient purine alkylation. Overall, the use of Tert-Butyl bromoacetate in conjunction with Cy2SI protecting group offers a powerful synthetic strategy for achieving regioselective and diastereoselective purine modifications with potential applications in the synthesis of nucleoside analogs and pharmaceutical compounds. 3
Safety
Tert-Butyl bromoacetate is a flammable liquid and vapor that poses significant health hazards. It is harmful if swallowed and can cause severe skin burns and eye damage upon contact. Additionally, it has the potential to cause skin and serious eye irritation, as well as respiratory irritation. Due to its dermatotoxic properties, it has the potential to cause skin burns. Therefore, it is crucial to handle Tert-Butyl bromoacetate with extreme care, ensuring proper protective measures are in place, such as using appropriate personal protective equipment and working in a well-ventilated area. Regular risk assessments and adherence to safety protocols are essential when dealing with this substance. 4
Reference
1. Toshima H, Fujino Y, Ichihara A, Koda Y, Kikuta Y. Syntheses of 3-Oxa-OPC and 2-Fluoro-OPC Homologues. Biosci Biotechnol Biochem. 1998;62(4):689-694.
2. Suzuki H, Kanai A, Uehara T, Guerra Gomez FL, Hanaoka H, Arano Y. Facile synthesis and evaluation of C-functionalized benzyl-1-oxa-4,7,10-triazacyclododecane-N,N',N″-triacetic acid as chelating agent for In-labeled polypeptides. Bioorg Med Chem. 2012 Jan 15;20(2):978-984.
3. Pal A, Salandria KJ, Arico JW, Schlegel MK, McLaughlin LW. 2,3-Dicyclohexylsuccinimide as a directing/protecting group for the regioselective glycosylation or alkylation of purines. Chem Commun (Camb). 2013 Apr 11;49(28):2936-2938.
4. Tert-butyl bromoacetate. European Chemicals Agency, EC / List no. 226-133-6.
Related articles And Qustion
Lastest Price from tert-Butyl bromoacetate manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 5292-43-3
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons

US $0.00-0.00/kg2025-04-21
- CAS:
- 5292-43-3
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons