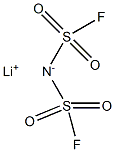
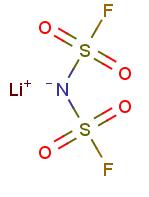
Lithium Bis(fluorosulfonyl)imide: Synthesis and properties
General description
Lithium Bis(fluorosulfonyl)imide (LiFSI), a new type of electrolyte solute with higher safety and stability, has officially ushered in a development opportunity. In recent years, with the state's support for the new energy vehicle industry, the new energy vehicle market has developed rapidly. With the decline of financial subsidies for new energy vehicles and the gradual enhancement of the market-driven effect, the lithium-ion power battery industry is rapidly moving towards high-quality development. Moreover, the state requires the lithium battery to develop in the direction of higher safety and higher energy density in the future, that is, the safety requirements for the electrolyte are greatly improved. Very recently, there has been a growing interest in Lithium Bis(fluorosulfonyl)imide as conducting salt and its ionic liquids as non-flammable solvents for Li (or Li-ion) batteries [1], after the recognition that pure ionic liquid electrolytes based on the FSI− anion, without any additives other than a lithium salt, are not only compatible with Li metal electrode but also with graphitized carbon electrode for Li-ion batteries, which was not previously seen with ILs based on other anions. Lithium Bis(fluorosulfonyl)imide was first claimed as conducting salt with good anticorrosive properties for Li-ion batteries in 1995 [2]; however, little attention was paid to it until recently, presumably due to the fact that it is rather difficult to prepare this salt with high purity in an unspecialized laboratory [3]. Although Lithium Bis(fluorosulfonyl)imide has been appreciated as conducting salt for Li-ion batteries for long times, little work has been done on its corrosive property towards Al. Only very recently, it has been reported that Lithium Bis(fluorosulfonyl)imide exhibited a corrosive potential as low as 3.3 V vs. Li+ /Li towards Al [4]. Its appearance is as follows:

Figure 1 Appearance of Lithium Bis(fluorosulfonyl)imide
Synthesis
1.01 standard stoichiometric amount of another ClSO2F was added to the fluorosulfonamide of Example 2, and then 1.01 standard stoichiometric amount of NH3 was added and kept at 50 °C. 2 hours. After cooling and expanding to atmospheric pressure, the formed NH4Cl is filtered out again. A brown solid is isolated, which is characterized by bis-fluorosulfonimide. The (crude) bissulfonimide was dissolved in isopropanol in a glass flask containing 2 times the stoichiometric amount of LiOH. After 3 hours at 75°C, the isopropanol was removed under vacuum with standard stoichiometric water. The yield of Lithium Bis(fluorosulfonyl)imide obtained after recrystallization in isopropanol was 159 g of Lithium Bis(fluorosulfonyl)imide with a purity of 99.99% and a melting point of 128-130°C [5].
Properties
The phase transition, thermal stability and pyrolysis behavior of the neat Lithium Bis(fluorosulfonyl)imide salt was investigated by DSC-TG-MS under argon flow [6]. It shows a melting point at 145 ℃ as a sharp endothermic peak with an onset at 135 ℃, both of which are obviously higher than the respective reported values of 132 and 124–128 ℃, indicating that Lithium Bis(fluorosulfonyl)imide is of high purity. It is thermally stable up to 200 ℃ without mass loss on TGA and detectable gaseous products on on-line MS. However, Lithium Bis(fluorosulfonyl)imide starts to decompose above 200 ℃, but shows only ≈ 3% mass loss from 200 to 300 ℃, during which SO2 (m/e = 64) is being detected as main gaseous products on on-line MS. The rate of decomposition becomes rapid from about 330 ℃, as evidenced by the two consecutive exothermic peaks on the DSC trace. The radicals for SO2 (m/e = 64) and NO2 (m/e = 46) are instantaneously detected as main signals by on-line MS above 300 ℃. The weight percentage of the residual is about 31%, which remains nearly constant to 520 ℃, the end of pyrolysis test. It is unlikely to be a singly pure LiF solid, as the weight percentage of the residual (31%) on TGA obviously deviates from the stoichiometric ratio of LiF/ Lithium Bis(fluorosulfonyl)imide, and needs to be identified in future. All above results clearly indicate that the thermal stability of Lithium Bis(fluorosulfonyl)imide is obviously higher than that for LiPF6 (107 ℃) measured under similar conditions.
[2]C. Michot, M. Armand, J.Y. Sanchez, Y. Choquette, M. Gauthier, United States Patent US 5916475 (1999).
[3]C. Michot, Canadian Patent CA 2527802 (2007).
[4]A. Abouimrane, J. Ding, I.J. Davidson, J. Power Sources 189 (2009) 693.
[5]Cui et al. Method for preparation of fluorinated conductive salts with application in lithium ion battery. China, CN111362843 A 2020-07-03.
[6]Han et al. Lithium bis(fluorosulfonyl)imide (LiFSI) as conducting salt for nonaqueous liquid electrolytes for lithium-ion batteries: Physicochemical and electrochemical properties. Journal of Power Sources 196 (2011) 3623–3632.
You may like
Related articles And Qustion
Lastest Price from Lithium Bis(fluorosulfonyl)imide manufacturers

US $0.00-0.00/KG2025-04-15
- CAS:
- 171611-11-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg
US $0.00-0.00/kg2025-04-04
- CAS:
- 171611-11-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton