Enhancing Battery Stability with Lithium Bis(fluorosulfonyl)imide as a Conductive Agent
General Description
Lithium bis(fluorosulfonyl)imide, a white crystalline substance, is widely used as a lithium salt in electrolytes for lithium-ion batteries. It offers high electrochemical stability and conductivity, making it crucial for battery technology. With its molecular structure and lack of stereocenters, it is suitable for advanced battery applications. Additionally, it serves as a rare earth Lewis acid catalyst and is utilized in the synthesis of chiral imidazolium salts. Lithium bis(fluorosulfonyl)imide finds application in clean energy devices such as lithium batteries and supercapacitors, as well as in ion liquids and as an antistatic agent. However, it poses risks to human health and the environment, requiring strict safety measures during handling and storage. Overall, lithium bis(fluorosulfonyl)imide plays a crucial role in advancing clean energy technologies and catalysis.

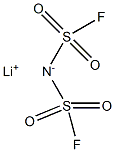
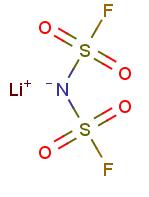
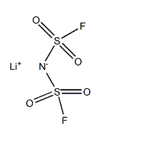
Figure 1. Lithium Bis(fluorosulfonyl)imide
Properties for Battery Technology
Lithium bis(fluorosulfonyl)imide, a white crystalline or powder substance, is utilized as a lithium salt in organic electrolytes for lithium-ion batteries. It exhibits high electrochemical stability and conductivity, making it an essential component in battery technology. With a molecular weight of 187.1 g/mol, it has no hydrogen bond donor and seven hydrogen bond acceptor counts. The compound has two rotatable bonds and a topological polar surface area of 86 Ų. Its exact mass is 186.93968459 g/mol, and it consists of 10 heavy atoms. The formal charge is zero, and the compound exhibits a complexity value of 235. Additionally, it contains no isotope atom count and no defined or undefined atom or bond stereocenters. Overall, lithium bis(fluorosulfonyl)imide's properties, including its molecular structure and lack of stereocenters, contribute to its suitability as a lithium salt in advanced battery applications due to its high stability and conductivity. 1
Diverse Applications in Various Industries
Lithium Bis(fluorosulfonyl)imide has various applications in different industries. It is primarily used in the production of electrolytes for lithium batteries and as a novel rare earth Lewis acid catalyst. Additionally, it can be utilized in the synthesis of chiral imidazolium salts through anion exchange reactions with corresponding trifluoromethanesulfonate salts. Lithium Bis(fluorosulfonyl)imide is an important fluorinated organic ionic compound with significant industrial applications in clean energy devices such as secondary lithium batteries, supercapacitors, and aluminum electrolytic capacitors. Its use extends to high-performance non-aqueous electrolytes, materials for chemical books, and new efficient catalysts. In terms of lithium battery electrolytes, Lithium Bis(fluorosulfonyl)imide serves as a conductive agent, helping to enhance the performance and stability of the batteries. It is also used in the production of ion liquids and as an antistatic agent. While its application in the medical field is relatively limited, it can be employed in pharmaceuticals. Overall, Lithium Bis(fluorosulfonyl)imide finds extensive use as an important component in lithium battery electrolytes and contributes to advancements in clean energy technologies and catalysis. 2
Safety
Lithium Bis(fluorosulfonyl)imide poses several safety hazards and should be handled with care. It is considered toxic and harmful if swallowed, as ingestion can lead to severe health issues. Additionally, exposure to Lithium Bis(fluorosulfonyl)imide can cause skin burns, skin irritation, and may even lead to allergic skin reactions. Contact with the eyes can result in serious damage, making it crucial to avoid eye exposure. Moreover, there are concerns about its potential impact on fertility and the unborn child, indicating the need for special precautions, especially for individuals who are pregnant or planning to conceive. Furthermore, the substance is harmful to aquatic life with long-lasting effects. Therefore, it is essential to prevent its release into the environment, as it could have a detrimental impact on aquatic ecosystems. Given these potential risks, proper safety measures must be implemented when dealing with Lithium Bis(fluorosulfonyl)imide. Protective equipment, including gloves, goggles, and appropriate clothing, should be worn to minimize the risk of skin and eye exposure. Additionally, handling procedures should be conducted in well-ventilated areas or using specialized fume hoods to prevent inhalation of vapors. Spill containment and clean-up protocols should be in place to avoid environmental contamination. In summary, Lithium Bis(fluorosulfonyl)imide presents various safety hazards, and it is crucial to adhere to strict safety protocols to mitigate potential risks to human health and the environment. 3
Reference
1. Lithium bis(fluorosulfonyl)imide. National Center for Biotechnology Information, 2023, PubChem Compound Summary for CID 86277430.
2. Judez X, Zhang H, Li C, González-Marcos JA, Zhou Z, Armand M, Rodriguez-Martinez LM. Lithium Bis(fluorosulfonyl)imide/Poly(ethylene oxide) Polymer Electrolyte for All Solid-State Li-S Cell. J Phys Chem Lett. 2017 May 4;8(9):1956-1960.
3. Lithium bis(fluorosulfonyl)imide. European Chemicals Agency, EC / List no. 924-516-9.
Related articles And Qustion
See also
Lastest Price from Lithium Bis(fluorosulfonyl)imide manufacturers

US $0.00-0.00/KG2025-04-15
- CAS:
- 171611-11-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg
US $0.00-0.00/kg2025-04-04
- CAS:
- 171611-11-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton