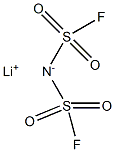
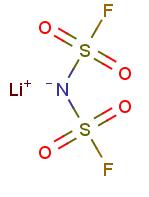
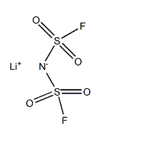
Application of lithium Bis(fluorosulfonyl)imide on lithium ion batteries
Lithium Bis(fluorosulfonyl)imide can be used as a lithium ion battery electrolyte additive in the electrolyte of rechargeable lithium battery, which can effectively reduce the high and low temperature resistance of the SEI layer formed on the surface of the electrode plate at low temperature, and reduce the lithium battery during the placement process. Capacity loss, thus providing high battery capacity and electrochemical performance of the battery, can also be used as a primary battery electrolyte; can be used as a polymerization catalyst; can also be used in the industrial field antistatic agent.
Over the past two decades, great efforts have been made to develop new lithium salts with improved chemical and/or electrochemical properties. Thus far, numerous weakly coordinating anions have been proposed as possible anion counterparts of lithium salts for Li-ion batteries, such as those containing nitrogen, phosphorus, carbon, or boron as central atom, large cluster-based anions, and aromatic heterocyclics. Very recently, there has been a growing interest in lithium bis(fluorosulfonyl)imide Li[N(SO2F)2] (LiFSI) as conducting salt and its ionic liquids as non-flammable solvents for Li (or Li-ion) batteries , after the recognition that pure ionic liquid electrolytes based on the FSI− anion, without any additives other than a lithium salt, are not only compatible with Li metal electrode but also with graphitized carbon electrode for Li-ion batteries , which was not previously seen with ILs based on other anions.
Ambient-temperature ionic liquids have attracted increasing attention in many fields including batteries, electroplating, synthetic and catalytic chemistry because of their diverse properties such as a wide electrochemical potential window, acceptable ionic conductivity, high thermal stability and negligible vapor pressure.
In particular, the application of these ionic liquids in lithium Liion secondary batteries has been considered in order to ensure safety by taking advantage of their lower flammability and lower reactivity than conventional organic electrolytes. However, the Liion batteries with ionic liquid electrolytes have some difficulties in their charge–discharge performance such as irreversibility of a carbon negative electrode.
Concerning this, someorganic additives can stabilize and protect an interface between a carbon negative electrode and ionic liquid phase against an undesirable irreversible reaction of ionic liquid components. Recently, Holzapfel et al. reported a reversible Li intercalation into an artificial graphite in a 1-M LiPF6 solution of 1-ethyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide (EMITFSI)containing 5 wt.% of vinylene carbonate (VC) as an organic solvent additive. Zheng et al. reported the effect of various organic solvent additives, including VC, on the cyclability of a graphitized negative electrode in an ionic liquid. In spite ofmany reports like them, however, there had been for long time no report on ambienttemperature ionic liquids that can provide reversibility of a graphitized negative electrode without both a halide ion and a solvent. We succeeded in finding novel electrolytes, bis(fluorosulfonyl) imide (FSI) anion-based ionic liquids, that provide a reversible capacity and high charge–discharge efficiency for a graphitized negative electrode without any additives. To apply the FSI based ionic liquids for practical purposes, however, it is necessary to clarify the effect and mechanism of the FSI anion on the negative electrode reactions.
References
[1]Sugimoto T , Atsumi Y , Kikuta M , et al. Ionic liquid electrolyte systems based on bis(fluorosulfonyl)imide for lithium-ion batteries[J]. Journal of Power Sources, 2009, 189(1):802-805.
[2]Han H B , Zhou S S , Zhang D J , et al. Lithium bis(fluorosulfonyl)imide (LiFSI) as conducting salt for nonaqueous liquid electrolytes for lithium-ion batteries: Physicochemical and electrochemical properties[J]. Journal of Power Sources, 2011, 196(7):3623-3632.
[3]Zhang H , Liu C , Zheng L , et al. Lithium bis(fluorosulfonyl)-imide/poly(ethylene oxide) polymer electrolyte[J]. Electrochimica Acta, 2014, 133:529-538.
Related articles And Qustion
See also
Lastest Price from Lithium Bis(fluorosulfonyl)imide manufacturers

US $0.00-0.00/KG2025-04-15
- CAS:
- 171611-11-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg
US $0.00-0.00/kg2025-04-04
- CAS:
- 171611-11-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton