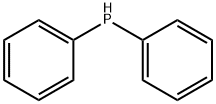
Diphenylphosphine: Applications in Organometallic Chemistry and Water Treatment
General Description
Diphenylphosphine are a type of chemical materials and fine chemicals with high added value. Because of their unique structure, properties and extensive application, they are widely used in catalysis, flame retardant materials, optical materials and other fields. The most frequently employed method for their preparations is treatment of electrophilic phosphorus reagents with organometallic reagents, which lacks tolerance for functional groups. In the 1980s, Hirao and co-workers described the first example of aryl and vinyl halide couplings with dialkyl phosphites under catalyst Pd(0). Following this, remarkable progress in the development of Csp2 −P bond formation by cross-coupling has been reported in the past decades, containing various methods via transition metal-catalyzed direct activation of the P-X(X = halogen, CN5 , B6 , Bi7 , N8 , O9 , S10 , Si11 etc.) and P−H4a, (Si4b ; Sn4c ) bond. This article will introduce the applications of diphenylphosphine.

Figure 1. Diphenylphosphine
Applications in Organometallic Chemistry
Regina Buffon and colleagues published a research paper, describes the synthesis of a cyclophosphazene-based diphenylphosphine ligand and a new Pd(0) complex. Infrared spectroscopy (ATR-IR), ESI+-MS, 31P, 1H and 13C NMR, Raman, WD-XRF, ICP-OES and TGA analysis show the coordination of two palladium atoms per unit of cyclophosphazene. A semiempirical calculation method was employed to find the lowest energy structure among the possible ones and Density Functional Theory (DFT) was used to optimize the found structure and obtain its bond angles, dihedral angles, bond lengths, atomic distances, and to calculate the vibrational spectrum (PBE/def2-TZVP(-f)). The new Pd complex showed activity in Suzuki-Miyaura cross-coupling reactions with halobenzenes and phenylboronic acid, tolerating different functional groups.1
Applications in Water Treatment2
Background
In recent years, increasing discharge of wastewater containing toxic organic dyes or heavy metals has presented a great threat to water/soil pollution and human health. As a result, there have been extensive studies to develop new and efficient methods to remove these toxins from water. Covalently linked porous hybrid polymers with large specific surface area, low density, and high thermal and chemical stabilities have shown a great deal of promise as efficient and recyclable adsorbents for various dyes and metal ions. Multifunctional and rigid cage silsesquioxanes with three-dimensional nanosized organic−inorganic structures represent interesting building blocks for the construction of hybrid porous polymers. A number of functional silsesquioxane-based porous hybrid polymers are developed using different synthetic methods, which include the Friedel−Crafts reaction, Heck reaction, Scholl coupling, Sonogashira cross-coupling, cationic polymerization, radical polymerization, and hydrosilation. There remains considerable space to explore new types and applications of silsesquioxane-based hybrid porous polymers.
Polymer frameworks functionalized with ferrocene moieties are well-known in literature and find applications in the fields of gas storage and electrocatalysis. The construction of porous materials incorporating ferrocene as an organometallic fragment endow the resulting structures with new properties for many prospective applications.
Efficiency in Water Treatment
Xiaoru Yang and Hongzhi Liu describes the synthesis of two porous hybrid polymers (abbreviated as DPPF-HPP and DPPOF-HPP) from the Friedel−Crafts reaction of octavinylsilsesquioxane with 1,1′-bis(diphenylphosphine)ferrocene (DPPF) and 1,1′-bis(diphenylphosphine oxide)ferrocene (DPPOF), respectively. DPPF-HPP and DPPOF-HPP possess surface areas of about 890 and 780 m 2 g−1, respectively, as well as similar pore structures of the coexisting micropores and mesopores. They are excellent materials for high adsorption of different dyes with adsorption capacities of 2280 mg g−1 for Congo Red and 1440 mg g−1 for Crystal Violet. DPPF-HPP also shows a strong affinity to adsorb Hg 2+ ions (300 mg g−1). These materials show no sign of degradation under repeated cycles and thus offer potential for wastewater treatment.
References:
[1] MARIA DAS G. DE O. E SILVA . Synthesis and characterization of a palladium(0) complex with cyclophosphazene bearing two diphenylphosphine ligands and application in Suzuki-Miyaura cross-coupling[J]. Inorganica Chimica Acta, 2018, 482: 1-952. DOI:10.1016/j.ica.2018.05.016.[2] XIAORU YANG H L. Diphenylphosphine-Substituted Ferrocene/Silsesquioxane-Based Hybrid Porous Polymers as Highly Efficient Adsorbents for Water Treatment[J]. ACS Applied Materials & Interfaces, 2019, 11 29: 25637-26528. DOI:10.1021/acsami.9b07874.
You may like
See also
Lastest Price from Diphenylphosphine manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 829-85-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt
US $0.00-0.00/kg2025-04-04
- CAS:
- 829-85-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton