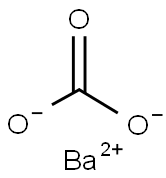
What is the decomposition reaction of Barium carbonate?
Barium carbonate has the empirical formula BaCO3 and a molecular weight of 197.37. It is a tasteless, odourless, heavy-white powder with a density of 4.2865. At about 1300°C, it decomposes into BaO and CO2. Its vapour pressure is negligible. Barium carbonate is almost insoluble in water. It is slightly soluble (1:1000) in water saturated with carbon dioxide, soluble in dilute hydrochloric or nitric acid or acetic acid and soluble in solutions of ammonium chloride nitrate.
Barium compounds gained access to the industry. Metallic barium aluminium/magnesium alloys are used as a getter in electron tubes and for the activation of electrodes. Moreover, lead/barium alloys that provide a high mechanical resistance are used as bearing metals. Different barium compounds are applied for several purposes: barium acetate is used as a mordant for the printing industry and as a catalyst for organic compounds. Barium carbonate is applied in manufacturing glassware, bricks, and ceramic materials. Barium hydroxide is used for glass fabrication, oil cleansing, and softening. Principal applications of the mainly occurring barium sulfate are found as x-ray contrast agents in the raw oil industry, the plastic and textile industry, and the paint industry.

An example of how effective barium carbonate is at precipitating the soluble salts in terra cotta clay. These two unglazed, cone 04-fired, mugs are made from the same clay, but the one on the left has 0.35% added barium carbonate.
The equilibrium vapour pressure of barium carbonate and the vacuum decomposition rates of the (001) face of its single crystal were measured by Basu et al. through torsion–effusion and torsion–Langmuir techniques, respectively. The free surface decomposition reaction rate was constant for product layers up to about 1 mm thick. The apparent activation enthalpy of decomposition for the reaction BaCO3(s)= BaO(s)+ CO2(g) is 225.9 kJ mol–1, which is less than the enthalpy of the equilibrium reaction, 252.1 kJ mol–1. The apparent activation entropy for the reaction is 53.5 J mol–1 K–1. This entropy is also less than the entropy of the equilibrium reaction, 146.6 J mol–1 K–1 in the temperature range studied[1].
Arvanitidis et al. investigated the decomposition reaction, BaCO3 (solid) = BaO (solid) + CO2 (gas), by thermogravimetric analysis (TGA) and differential thermal analysis (DTA) methods. Both shallow powder beds and densely compacted spheres of the carbonate were employed. In the case of the shallow powder beds, TGA and DTA were carried out simultaneously. The DTA curves showed that BaCO3 exhibited two phase transformations, the transformation of orthorhombic to hexagonal occurring at 1079 K and that of hexagonal to cubic at 1237 K. The activation energy and the forward reaction rate constant of the decomposition of BaCO3 were evaluated from the thermogravimetric results of the powder beds. The activation energy of the decomposition was found to be 305(± 14) kJ • mole−1. The experimental results obtained with the compacted spheres were compared with those corresponding to the powder beds. After the initial stages, the formation of liquid due to the eutectic reaction between BaCO3 and BaO appears to play an essential role in the reaction kinetics[2].
[1] T. K. Basu, A. Searcy. “Kinetics and thermodynamics of decomposition of barium carbonate.” Journal of the Chemical Society, Faraday Transactions 85 1 (1976): 1889–1895.
[2] I. Arvanitidis, S. Seetharaman, Du. Siche. “A study of the thermal decomposition of BaCO3.” Metallurgical and Materials Transactions B 64 1 (1996): 409–416.
References:
[1] I. ARVANITIDIS S S Du Siche. A study of the thermal decomposition of BaCO3[J]. Metallurgical and Materials Transactions B, 1996, 64 1: 261-272. DOI:10.1007/BF02914905.[2] T. K. BASU A S. Kinetics and thermodynamics of decomposition of barium carbonate[J]. Journal of the Chemical Society, Faraday Transactions, 1976, 85 1: 158-159. DOI:10.1039/F19767201889.
You may like
Related articles And Qustion
Lastest Price from Barium carbonate manufacturers

US $8.00/KG2025-06-09
- CAS:
- 513-77-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 ton

US $10.00/KG2025-04-21
- CAS:
- 513-77-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt