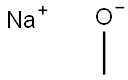The brief introduction of Sodium Methoxide
Introduction
Sodium methoxide (Also known as Sodium methanolate, Methoxy sodium, or Sodium methylate) is the simplest sodium alkoxide.

It reacts with water to form sodium hydroxide, a corrosive material, and methyl alcohol, a flammable liquid. The heat from this reaction may be sufficient to ignite surrounding combustible material or the sodium methylate itself if the water is in only small amounts. It is used to process edible fats and oils and to make other chemicals. The formula CH3ONa is in two forms: white solid powder, with 99% purity and liquid solution in methanol, with 30% purity.
Preparation method
Existing NaOCH3 synthesis technologies include the reactordistillation-distillation system and reactive distillation-distillation scheme.
One method to prepare CH3ONa is reacting metallic sodium with methanol because of the high cost of metallic sodium and hydrogen release in the process; another preparation method by sodium hydroxide and methanol is more widely used in continuous production. It is usually carried out in a reactive distillation column with a large amount of gaseous anhydrous methanol fed into the bottom of the column. Gaseous methanol contacts countercurrent with sodium hydroxide-methanol solution, removes water from the reaction phase, and increases the production of CH3ONa [1]. The chemical reaction can be represented as follows:
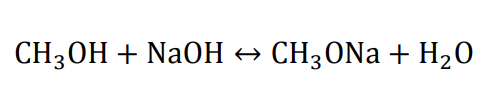
Uses
Sodium methoxide is a colourless, flammable liquid used to manufacture Formaldehyde and Acetic acid in chemical synthesis, antifreeze, and as a solvent. Ingestion of methanol is toxic and may cause blindness.
It is a high-performance alkoxide catalyst primarily used in biodiesel production from waste cooking oil, chicken fat, palm oil, and canola oil as an intermediary in 1G-biodiesel and enzymatic saccharification enhancer[2]. In biodiesel production, 25-30 wt% NaOCH3 in methanol (CH3OH) is used as a homogeneous catalyst for transesterification reaction. NaOCH3 is a more effective alternative to alkaline metal hydroxides to improve the yield and purity of biodiesel. It is being extensively investigated as an efficient catalyst for biodiesel production, aiming to lower the reaction temperature, reduce the reaction time and increase biodiesel yields. NaOMe is a simple catalyst that could provide superior accessibility to a wide variety of functionalized amides, including peptides, through direct amination of esters in an atom-economical and environmentally benign way[3].
[1] Ohshima, Takashi et al. “Sodium methoxide: a simple but highly efficient catalyst for the direct amidation of esters†.” Chemical Communications 44 (2012): 5434–5436.
[2] Natthiyar Aeamsuksai. “Comparison of Different Synthesis Schemes for Production of Sodium Methoxide from Methanol and Sodium Hydroxide.” AlKhawarizmi Engineering Journal 67 1 (2020).
[3] Meng Xiong, D. Shao, C. Wang. “Preparing Sodium Methoxide from Sodium Hydroxide by Reaction Coupling with Separation Processes.” Advanced Materials Research 5 1 (2014): 101–105.
References:
[1] MENG XIONG D S C Wang. Preparing Sodium Methoxide from Sodium Hydroxide by Reaction Coupling with Separation Processes[J]. Advanced Materials Research, 2014, 5 1: 17-33. DOI:10.4028/WWW.SCIENTIFIC.NET/AMR.986-987.101.[2] OHSHIMA T, HAYASHI Y, AGURA K, et al. Sodium methoxide: a simple but highly efficient catalyst for the direct amidation of esters?[J]. Chemical Communications, 2012, 44: Page 5381 to 5528. DOI:10.1039/C2CC32153J.
[3] NATTHIYAR AEAMSUKSAI. Comparison of Different Synthesis Schemes for Production of Sodium Methoxide from Methanol and Sodium Hydroxide[J]. AlKhawarizmi Engineering Journal, 2020, 67 1: 741-758. DOI:10.4186/ej.2020.24.6.63.
See also
Lastest Price from Sodium Methoxide manufacturers

US $1.00/kg2025-04-21
- CAS:
- 124-41-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/Kg2025-04-21
- CAS:
- 124-41-4
- Min. Order:
- 1Kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons