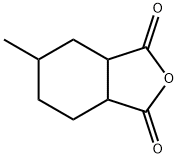
Synthesis of Hexahydro-4-methylphthalic anhydride
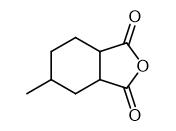
Synthetic routes
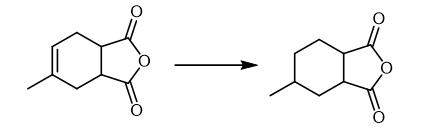
Fig. 2 The synthetic method 1 of Hexahydro-4-methylphthalic anhydride.
Take
up 4-methyl-1,2,3,6-tetrahydrophthalic anhydride (3 mmol) in 1.5 mL of
AcOEt (2 M). Fill the mixture into loop of 1 mL. Take up maleic
anhydride (3.3 mmol) in 1.5 mL of AcOEt (2.2 M). Fill the mixture into
another loop of 1 mL. Inject the two loops simultaneously into streams
of AcOEt at 0.25 1 mL min-1 (each pump at 0.125 1 mL min-1).
Plug the mixture to met at a T-piece before passing through a coil
reactor (10 mL, 40 minutes) at 140°C. Pressurize the mixture by a 10 bar
back-pressure regulator. Collect the cycloaddition product in a flask
of 20 mL. Pump the mixture directly by an AZURA P4. Pressurize 1S pump
using a flow rate of 1 mL min-1 through the tube-in-tube
reactor, at 15 bar of hydrogen, in order to saturate the liquid stream
with H2. Pass the reaction mixture containing the starting material and
solubilized H2 subsequently through the cartridge (6.6 mm
i.d. × 50.0 mm length) containing Pd-C catalyst (30% Pd-C, 750 mg, void
volume approximately 1 mL). Pressurize the hydrogenation system by a 16
bar back-pressure regulator for 160 minutes. Place the pump inlets in
the same round bottomed flask. Recycle the same reaction mixture through
the system until complete hydrogenation of the starting materials,
which varied from 80 to 510 minutes. Monitor the reaction evolution by
gas chromatography-mass spectrometry.
1H NMR (250 MHz, CDCl3) δ 3.25-2.96 (m, 2H), 2.38-2.08 (m, 2H), 1.76-1.58 (m, 2H), 1.51-1.21 (m, 2H), 0.99-0.87 (m, 4H). 13C NMR (63 MHz, CDCl3) δ 172.88, 172.61, 40.86, 40.32, 34.45, 30.34, 29.22, 21.96, 21.29. IR (ATR, cm-1): 2359 (w), 2340 (w), 1694 (s), 1452 (w), 1413 (w), 1336 (w), 1293 (w), 1251(w) cm-1. HRMS (ESI+) m/z: Calcd. for C9H12O3H+ [M + H]+ 169.0859; found: 169.0855 [1].
Application
In the reaction of glycidyl phenyl ether and hexahydro-4-methylphthalic anhydride
The catalytic effects of 1,5,7-Triazabicyclo[4.4.0]dec-5-ene (TBD) with 2-methylimidazole-intercalated alpha-zirconium phosphate (alpha-ZrP center dot 2MIm) in the reaction of glycidyl phenyl ether (GPE) and hexahydro-4-methylphthalic anhydride (MHHPA) were investigated. The reaction did not proceed within 1 h at 60 degrees C. On increasing the temperature to 100 degrees C, the conversion reached 93% for 1 h. Without the addition of TBD, the conversion was 67% at 100 degrees C for 1 h. Under storage conditions at 25 degrees C for 7 days, the conversion of GPE was only 18%. The curing behavior of 2,2-bis(4-glycidyloxyphenyl)propane (DGEBA) and MHHPA in the presence of TBD with alpha-ZrP center dot 2MIm was evaluated by differential scanning calorimetry. The addition of TBD with alpha-ZrP center dot 2MIm as a latent thermal initiator, the storage stability was maintained and the reaction proceeded rapidly under heating conditions [2].
The capabilities of imidazoles-intercalated alpha-zirconium phosphate (alpha-ZrPimidazole): imidazol (alpha-ZrPIm), 2-methylimidazole (alpha-ZrP2MIm), and 2-ethyl-4-methylimidazole (alpha-ZrP2E4MIm) as latent thermal initiators were examined by the copolymerization of glycidyl phenyl ether (GPE) and hexahydro-4-methylphthalic anhydride (MHHPA) with the imidazoles-intercalated-zirconium phosphate at varying temperatures for one-hour periods. Polymerization was not observed until the reactants were heated to 100 degrees C or above. Increasing the temperature, polymerization in the presence of alpha-ZrPIm, alpha-ZrP2MIm, or alpha-ZrP2E4MIm proceeded at 140 degrees C for 1 h with over 90% conversion. The thermal stabilities of alpha-ZrPIm, alpha-ZrP2MIm, and alpha-ZrP2E4MIm in the reaction at 40 degrees C for 264 h were tested. With alpha-ZrP2MIm, the conversion was less than 15% up to 96 h. In the cases of alpha-ZrPIm and alpha-ZrP2E4MIm, the conversion reached less than 15% at 264 h. The thermal stabilities of alpha-ZrPIm, alpha-ZrP2MIm, and -ZrP2E4MIm at 40 degrees C were superior to those of the commercially available thermal latent initiators: HX-3088 and HX-3722 [3].
The latent catalytic abilities of tertiary amines-intercalated at-zirconium phosphate [(alpha-ZrP center dot amine): 1,4-diazabicyclo[2,2,2]octane (alpha-ZrP center dot DABCO) and 1,8-diazabicyclo[5,4,0]undec-7-ene (alpha-ZrP center dot DBU)] were examined by copolymerization of glycidyl phenyl ether (GPE) and hexahydro-4-methylphthalic anhydride (MHHPA) at varying temperatures for 1 h periods. Polymerization was not observed until the reactants were heated to 100 degrees C or above. Upon increasing the temperature, the conversion factors of GPE increased such that, at 140 degrees C, both conversions were over 90% for alpha-ZrP center dot DABCO and alpha-ZrP center dot DBU. The thermal stabilities of GPE and MHHPA with the catalysts at 40 degrees C for 144h were tested: GPE with alpha-ZrP center dot DBU achieved conversions of 9%. The reaction in the presence of alpha-ZrP center dot DABCO did not proceed at 40 degrees C for 144 h [4].
As a curing agent
The curing kinetics of the hexahydro-4-methylphthalic anhydride (MHHPA)/diglycidyl 1,2-cyclohexane dicarboxylate (CY184) epoxy resin system and MHHPA/CY184 epoxy/episulfide resin system (containing 2 mass% DMP-30 as an accelerator) was comparatively investigated by non-isothermal differential scanning calorimetry with a model-fitting Malek method and a model-free advanced isoconversional method of Vyazovkin, and the curing behavior was discussed based on the proposed curing mechanism. The results indicated that both of the MHHPA/CY184 epoxy resin system and MHHPA/CY184 epoxy/episulfide resin system fitted estak-Berggren model. The activation energy of MHHPA/CY184 epoxy/episulfide resin system was lower than that of MHHPA/CY184 epoxy resin system, suggesting that the episulfide resin has higher reactivity and can accelerate the reaction. The value of m in the kinetic model equation in MHHPA/CY184 epoxy/episulfide resin system is much smaller than that in MHHPA/CY184 epoxy resin system, indicating, unlike the MHHPA/CY184 epoxy resin system, MHHPA/CY184 epoxy/episulfide resin system has much less autocatalytic effect [5].
References
[1] Galaverna R, Fernandes L P, Browne D L, et al. Continuous flow processing as a tool for the generation of terpene-derived monomer libraries[J]. Reaction Chemistry & Engineering, 2019, 4(2): 362-367.
[2] Shimomura O, Sasaki S, Kume K, et al. Acceleration effects of 1, 5, 7‐triazabicyclo [4.4. 0] dec‐5‐ene with 2‐methylimidazole‐intercalated α‐zirconium phosphate as a latent thermal initiator in the reaction of glycidyl phenyl ether and hexahydro‐4‐methylphthalic anhydride[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2019, 57(24): 2557-2561.
[3] Shimomura O, Tokizane K, Nishisako T, et al. Imidazoles-intercalated α-zirconium phosphate as latent thermal initiators in the reaction of glycidyl phenyl ether (GPE) and hexahydro-4-methylphthalic anhydride (MHHPA)[J]. Catalysts, 2017, 7(6): 172.
[4] Shimomura O, Nishisako T, Yamaguchi S, et al. DABCO-and DBU-intercalated α-zirconium phosphate as latent thermal catalysts in the copolymerization of glycidyl phenyl ether (GPE) and hexahydro-4-methylphthalic anhydride (MHHPA)[J]. Journal of Molecular Catalysis A: Chemical, 2016, 411: 230-238.
[5] Zhang C, Liu X, Cheng J, et al. Study on curing kinetics of diglycidyl 1, 2-cyclohexane dicarboxylate epoxy/episulfide resin system with hexahydro-4-methylphthalic anhydride as a curing agent[J]. Journal of Thermal Analysis and Calorimetry, 2015, 120(3): 1893-1903.
See also
Lastest Price from Hexahydro-4-methylphthalic anhydride manufacturers

US $0.00-0.00/KG2025-04-04
- CAS:
- 19438-60-9
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton
US $0.00-0.00/KG2023-11-13
- CAS:
- 19438-60-9
- Min. Order:
- 1KG
- Purity:
- ≥98.0
- Supply Ability:
- 71000