Synthesis and Application of (R)-4-Propyldihydrofuran-2(3H)-one
Generally speaking
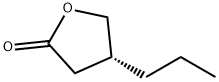
(R)-4-Propyldihydrofuran-2(3H)-one is a yellow oily liquid, slightly soluble in chloroform, methanol and ethanol. It needs to be stored at 2-8°C. (R)-4-Propyldihydrofuran-2(3H)-one is an organic synthesis intermediate and a pharmaceutical intermediate.
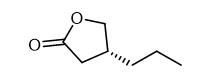
Fig. 1 The structure of (R)-4-Propyldihydrofuran-2(3H)-one.
Synthetic routes
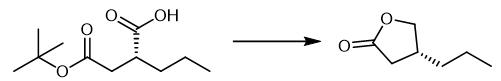
Fig. 2 The synthetic method 1 of (R)-4-Propyldihydrofuran-2(3H)-one.
Charge a 20 L double jacketed reactor equipped with a mechanical stirrer and a condenser with (R)-2-propylsuccinic acid 4-tert-butyl ester (1000.6 g) and toluene (8.5 L, 8.5 L/kg). Cool the solution to -5.1 °C under nitrogen flow. Add triethylamine (561.5 g, 5.55 mol, 1.2 equivalents) to the mixture maintaining the temperature between -6.0 °C and -5.1 °C over a period of 35 minutes. Add ethyl chloroformate (551.9 g, 5.09 mol, 1.1 equivalents) to the mixture maintaining the temperature between -6.6 °C and -3.4 °C over a period of 38 minutes. Stir the heterogeneous reaction mixture for 1 hour at -5 °C. Warm the reaction mixture to 20 °C. Filter the mixture to remove the triethylammonium chloride salt. Wash the cake with toluene (1.5 L, 1.5 L/kg) at 20 °C. Transfer the filtrate into the reactor. Cool the solution to -18.7 °C. Add powdered sodium borohydride (349.8 g, 9.25 mol, 2.0 equivalents) one pot to the mixture at -18.5 °C under nitrogen flow. Transfer methanol (2 L, 2 L/kg) dropwise in order to the mixture to maintain the temperature below -18 °C and to limit the formation of foaming (8 hours 40 minutes addition time between -21.4 °C and - 19.1 °C). Stir the reaction mixture for minimum 1 hour at -20 °C (15 hours 15 minutes, overnight). Add HCl 4M (2 L, 2 L/kg) dropwise in order to the mixture at -21.4 °C, under nitrogenflow to maintain the temperature below -18 °C and to limit the formation of foaming (5 hours 20 minutes addition time between -21.9 °C and -21.5 °C). Warm the reaction mixture to 20 °C. Add water (5 L, 5 L/kg) to the mixture until dissolution of salts. Discard the aqueous layer. An additional water wash (4 L, 4 L/kg) is done. Add trifluoroacetic acid 95% v/v (0.1 L, 0.1 L/kg, ~ 0.27 equivalent) one pot to the mixture at 25 °C. Stir the reaction mixture until the completion of the reaction (~ 1 hour). Wash the organic layer with water (2 × 3 L, 2 × 3 L/kg). Evaporate the organic layer. 1H NMR (CDCl3, 400 MHz) 4.42 (tapp, J = 8.0 Hz, 1 H) 3.93 (tapp, J = 8.0 Hz, 1 H) 2.65-2.54 (m, 2 H) 2.19 (dd, J = 16.3, 7.3 Hz, 1 H) 1.48-1.44 (m, 2 H) 1.40-1.30 (m, 2 H) 0.95 (t, J = 7.1 Hz, 3 H) ppm. 13C NMR (CDCl3, 100 MHz) 177.3, 73.4, 35.4, 35.2, 34.5, 20.5, 13.9 ppm [1].
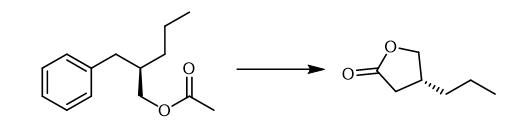
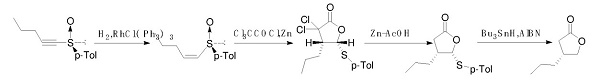
Fig. 3 The synthetic method 2 of (R)-4-Propyldihydrofuran-2(3H)-one.
Add a solution of H5IO6 (24.429 g, 107.17 mmol) in water (43 mL) to a solution of (R)-2-benzylpentyl acetate (1.180 g) in CCl4 (27 mL) and CH3CN (27 mL) at 0 °C. Add RuCl3·nH2O (0.222 g, 1.07 mmol) slowly to the mixture at 0 °C. Stir the resultant mixture vigorously for 20 hours at room temperature. Quench the reaction with Et2O (50 mL) at 0 °C. Stir the mixture for 30 minutes. Extract the mixture with Et2O (3 × 50 mL). Wash the combined organic layers with brine (30 mL). Dry the combined organic layers over anhydrous Na2SO4. Filter the combined organic layers. Remove the solvent. Dissolve the oil in aqueous NaOH (1 mol L-1, 40 mL). Stir the resulting solution overnight at room temperature. Wash the solution with Et2O (30 mL). Acidify the solution with aqueous HCl (6 mol L-1, 15 mL) at 0 °C. Stir the mixture overnight at room temperature. Saturate the aqueous layer with NaCl. Extract the mixture with Et2O (5 × 40 mL). Wash the combined organic layers with water, saturated aqueous Na2S2O3 (30 mL) and brine (30 mL). Dry the combined organic layers over anhydrous Na2SO4. Filter the combined organic layers. Remove the solvent. Purify the residue by column chromatography on silica gel (hexane/acetone = 3/1) and distillation (162-209 °C/36 mmHg) [2].
Application
For the synthesis of Brivaracetam
Epilepsy, commonly known as "sheep horn wind" or "sheep epilepsy", is a kind of abnormal movement, sensation, consciousness, spirit and autonomic function caused by abnormal paroxysmal discharge of brain neurons caused by various reasons. disease. The overall prevalence of epilepsy in China was 7.0%, the annual incidence was 28.8/100,000, and the prevalence of active epilepsy with seizures within 1 year was 4.6%. Brivaracetam, a racetam derivative, is a novel high-affinity synaptic vesicle protein 2A ligand, which can inhibit neuronal voltage-dependent sodium channels and play an anti-epileptic effect. Brivaracetam is a third-generation antiepileptic drug developed by Belgian UCB (UCB). Using (R)-4-propyl-dihydrofuran-2-one as raw material, brivaracetam is prepared through the steps of ring-opening, halogenation, condensation, and ring-closing [3].
References
[1] Schule A, Merschaert A, Szczepaniak C, et al. A biocatalytic route to the novel antiepileptic drug brivaracetam[J]. Organic Process Research & Development, 2016, 20(9): 1566-1575.
[2] Kawasaki M, Kato D, Okada T, et al. Synthesis and olfactory evaluation of optically active β-alkyl substituted γ-lactones and whiskey lactone analogues[J]. Tetrahedron, 2020, 76(12): 130984.
[3] Wang Y, Guo J, Luo M. Preparing brivaracetam comprises performing ring-opening reaction (R)-4-propyldihydrofuran-2(3H)-one, and performing ring-closure reaction with (2S)-2-aminobutyramide[P]. Faming Zhuanli Shenqing, 113943240, 2022.
Related articles And Qustion
See also
Lastest Price from (R)-4-Propyldihydrofuran-2(3H)-one manufacturers
US $0.00-0.00/mg2025-03-04
- CAS:
- 63095-51-2
- Min. Order:
- 10mg
- Purity:
- 98%
- Supply Ability:
- 500mg
US $0.00/KH2024-06-16
- CAS:
- 63095-51-2
- Min. Order:
- 5KH
- Purity:
- 99
- Supply Ability:
- 10Tons