The Rise of (R)-dihydro-4-propyl-2(3H)-furanone in Specialty Chemical Applications
Introduction
The chemical compound (R)-dihydro-4-propyl-2(3H)-furanone, with its distinct structural properties and functionalities, has increasingly become a focal point in the realm of synthetic organic chemistry. This compound is noteworthy not only for its unique chemical architecture but also for its utility across various industrial sectors. It exhibits significant potential due to its configurational specificity and the presence of a functional lactone ring, which enables a range of chemical reactions crucial for creating complex molecules. As a result, this compound has drawn significant attention for its application in creating innovative pharmaceuticals, eco-friendly agrochemicals, and novel materials. Moreover, its enantioselective synthesis poses both a challenge and an opportunity for chemists, fostering advancements in asymmetric catalysis and green chemistry practices. These developments underline the compound's growing importance in scientific research and industrial application, promising new possibilities in various fields of chemistry[1].

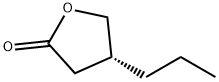
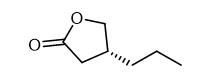
Figure 1 Characteristics of (R)-dihydro-4-propyl-2(3h)-furanone
Synthesis
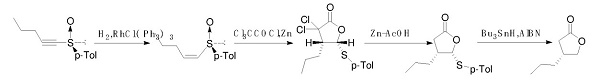
The synthetic route to (R)-dihydro-4-propyl-2(3H)-furanone is primarily centered around the formation of the furanone ring, which is typically achieved through cyclization reactions. Starting from simple ketones and aldehydes, the synthesis involves key steps such as condensation, cyclization, and subsequent reduction processes. These steps are carefully optimized to ensure the retention of the (R)-configuration at the propyl substitution, which is crucial for the compound's bioactivity and functionality. Advanced techniques such as asymmetric catalysis may be employed to enhance the yield and selectivity of the desired enantiomer.
Main Components
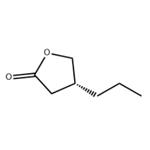
At its core, (R)-dihydro-4-propyl-2(3H)-furanone consists of a five-membered lactone ring incorporating an oxygen atom and a propyl side chain in an (R)-configuration. The molecular structure also features a ketone group, which plays a critical role in its chemical reactivity and further functionalization. The presence of these functional groups makes the compound versatile for modifications and useful in a range of chemical synthesis applications. Additionally, the ketone group provides a site for nucleophilic attack, facilitating various reactions such as reductions and Grignard reactions. This reactivity enhances the compound's utility in creating complex derivatives used in medicinal chemistry and materials science.
Applications
The applications of (R)-dihydro-4-propyl-2(3H)-furanone are diverse, spanning from pharmaceuticals to flavor and fragrance industries. In pharmaceuticals, its use as a synthetic intermediate for the preparation of more complex molecular entities is invaluable, particularly in the synthesis of new molecules with potential therapeutic effects. In the flavor and fragrance industry, this compound is cherished for its ability to impart a unique, warm, and subtly sweet aroma, making it an ideal component in the formulation of various fragrances and flavoring agents. Beyond these industries, (R)-dihydro-4-propyl-2(3H)-furanone finds applications in agricultural chemistry as a precursor to pheromone-based insect attractants. Its structural versatility also allows for exploration in the synthesis of organic light-emitting diodes (OLEDs) and other electronic materials, where its properties can be harnessed to improve performance and sustainability. These broad applications underscore its significant role in scientific and industrial advancements.
Storage Methods
Storing (R)-dihydro-4-propyl-2(3H)-furanone requires specific conditions to maintain its chemical integrity and prevent degradation. The compound should be stored in a cool, dry place, away from direct sunlight and moisture. It is typically kept in tightly sealed containers under inert atmospheres, such as nitrogen or argon, to avoid any oxidative degradation. Proper storage not only preserves the quality but also extends the shelf life of the compound, which is crucial for its effective use in industrial applications. Additionally, fluctuations in temperature should be minimized to prevent any adverse reactions that could compromise the compound’s stability and purity.
Conclusion
As the chemical industry continues to evolve, the significance of compounds like (R)-dihydro-4-propyl-2(3H)-furanone becomes increasingly apparent. Its versatile applications and unique properties make it a valuable asset in the arsenal of chemists and researchers dedicated to developing new materials and pharmaceutical agents. With ongoing advancements in synthetic techniques and an increasing understanding of its properties, (R)-dihydro-4-propyl-2(3H)-furanone is set to play a pivotal role in the future of chemical research and industry[2].
![Article illustration]() References
References
[1]Hentzer M, Riedel K, Rasmussen T B, et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound[J]. Microbiology, 2002, 148(1): 87-102.
[2]Baveja J K, Li G, Nordon R E, et al. Biological performance of a novel synthetic furanone-based antimicrobial[J]. Biomaterials, 2004, 25(20): 5013-5021.
You may like
Related articles And Qustion
Lastest Price from (R)-4-Propyldihydrofuran-2(3H)-one manufacturers
US $0.00-0.00/mg2025-03-04
- CAS:
- 63095-51-2
- Min. Order:
- 10mg
- Purity:
- 98%
- Supply Ability:
- 500mg
US $0.00/KH2024-06-16
- CAS:
- 63095-51-2
- Min. Order:
- 5KH
- Purity:
- 99
- Supply Ability:
- 10Tons