L-Cysteine Base: A Cornerstone in Chemical Synthesis and Applications
Introduction
L-cysteine base, a semi-essential amino acid, holds a pivotal position in the chemical and pharmaceutical industries due to its unique structural characteristics and functional properties. As an amino acid, it serves not only as a building block of proteins but also plays a crucial role in various metabolic processes. This sulfur-containing amino acid is particularly significant because of its ability to form disulfide bonds, which are essential for the structural integrity and function of many proteins. Additionally, L-cysteine is involved in numerous metabolic pathways, including the synthesis of glutathione, a major antioxidant that protects cells from oxidative stress and toxins. Its versatility and functional importance make the L-Cysteine Base a critical focus of study for biochemists and pharmacologists alike.

Figure 1 Characteristics of L-Cysteine Base
Synthesis of L-Cysteine Base
The synthesis of L-Cysteine Base primarily involves two major routes: the extraction from human hair or animal feathers and the bio-synthesis using microbial fermentation. The latter is gaining prominence due to ethical considerations and scalability. Microbial fermentation typically employs bacteria such as Escherichia coli that are genetically engineered to optimize the amino acid’s production. This method not only ensures a higher yield but also aligns with sustainable practices, as it reduces dependency on animal sources and minimizes waste.
Main Components
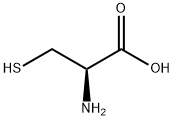
L-cysteine base primarily consists of the amino acid L-Cysteine itself, with a chemical structure characterized by a thiol side chain. This thiol group is reactive and serves as a functional component in various biochemical reactions, including redox reactions within cells. The purity of the L-Cysteine Base is critical for its effectiveness, and it is often assessed by high-performance liquid chromatography (HPLC) to ensure that it meets the stringent requirements of the pharmaceutical and food industries. The molecular structure of L-Cysteine allows it to act as a precursor in the synthesis of other significant biochemicals, such as taurine and coenzyme A, further underlining its importance in both metabolic and physiological contexts. This versatility makes L-Cysteine indispensable in research and industry applications.
Applications of L-Cysteine Base
The applications of L-Cysteine Base are vast and diverse. In the pharmaceutical sector, it is used to manufacture drugs that treat conditions like acetaminophen poisoning, where it replenishes glutathione in the liver, helping to detoxify harmful substances. In the food industry, L-Cysteine Base is a popular additive used as a dough conditioner in bakery products. It helps improve the texture and elasticity of dough by breaking disulfide bonds in gluten. Additionally, L-Cysteine plays a critical role in flavor production, especially in the production of meat flavors through Maillard reactions. Beyond these, L-Cysteine is crucial in cosmetic formulations, enhancing anti-aging properties by promoting collagen synthesis and skin repair. Its broad utility underscores its significance in diverse commercial and medical applications.
Storage Methods
Proper storage of the L-Cysteine Base is essential to maintain its efficacy and stability. It should be stored in a cool, dry place away from light, as it is sensitive to air and moisture, which can lead to oxidation of the thiol group, rendering it less effective. In its powdered form, L-Cysteine should be kept in tightly sealed containers under an inert atmosphere, preferably nitrogen, to prevent oxidation. The recommended storage temperature is typically below 25°C, with humidity control to prevent clumping and degradation.
Conclusion
L-Cysteine Base is an indispensable compound in the realms of chemistry and medicine, with a broad spectrum of applications that underline its significance. From its synthesis through environmentally friendly methods to its critical roles in health and industry, L-Cysteine Base exemplifies how a simple amino acid can have widespread impacts. As research continues to uncover new uses and improve existing methodologies, the importance of L-Cysteine Base in scientific and industrial applications is expected to grow, highlighting the need for ongoing investment in its production and application technologies.
![Article illustration]() References
References
[1]Kallen R G. Mechanism of reactions involving Schiff base intermediates. Thiazolidine formation from L-cysteine and formaldehyde[J]. Journal of the American Chemical Society, 1971, 93(23): 6236-6248.
[2]Carson J F, Boggs L E. The synthesis and base-catalyzed cyclization of (+)-and (-)-cis-S-(1-propenyl)-L-cysteine sulfoxides[J]. The Journal of Organic Chemistry, 1966, 31(9): 2862-2864.
Related articles And Qustion
Lastest Price from L-Cysteine manufacturers

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 52-90-4
- Min. Order:
- 1KG
- Purity:
- 98.0%-101.0%; AJI
- Supply Ability:
- 10 TONS

US $0.00/kg2025-04-21
- CAS:
- 52-90-4
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 10Ton