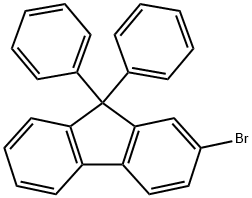
Synthesis of 2-Bromo-9,9-diphenylfluorene
Generally speaking
2-Bromo-9,9-diphenylfluorene is an organic fluorene compound that can be used as a pharmaceutical intermediate, and it is also an important intermediate for the synthesis of optoelectronic materials. Its molecular formula is C25H17Br and its melting point is 217.0 to 221.0 °C.
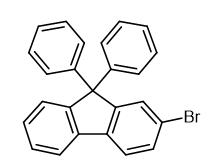
Fig. 1 The structure of 2-Bromo-9,9-diphenylfluorene.
Synthetic routes
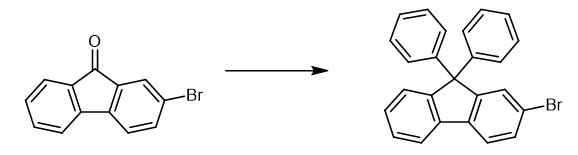
Fig. 2 The synthetic method 1 of 2-Bromo-9,9-diphenylfluorene.
2-Bromo-9H-fluoren-9-one (2g; 7.7 mmol) was dissolved in 50 mL of anhydrous tetrahydrofuran in a 100mL two-neck round-bottom flask that was equipped with a condenser and a magnetic stirrer. 2M phenylmagnesium chloride in THF (5.8 mL; 11.6 mmol) was added at room temperature. As the phenylmegnesium chloride was added, the solution slowly turned brown. It was heated and refluxed for 3 hours. Excess Grignard reagent was terminated by addition of methanol. After deionized water (100 mL) was added, the reaction mixture was extracted with ethyl acetate (50 mL X3). The combined organic layers were dried over anhydrous magnesium sulfate, and filtered off. The filtrate was condensed and dried under vacuum conditions to yield 2.6g of yellow viscous gel. After it had been dried in a vacuum, the viscous gel was directly dissolved in 30 mL benzene. The mixed solution was added dropwise to a solution of trifluoromethanesulfonic acid (1.8 g; mmol) in benzene. During the addition, the solution became red and then bleached. The reaction mixture was heated and refluxed for 6 hours. Aqueous sodium carbonate (1M, 50 mL) was added to neutralize the reaction solution when it recovered to room temperature. The solution was then extracted with ethyl acetate (50mL X 3). The combined organic solution was dried over anhydrous potassium carbonate, filtered off and condensed under vacuum conditions. It was further purified by column chromatography on silica gel using n-hexane as a diluent. Product 20 (2.0g) was obtained as a white powder in a yield of 65%. 2-Bromo-9,9-diphenyl-9H-fluorene, yield (2.0g) 65%. 1H NMR (300MHz, CDCl3, δ) 7.18-7.42(m, 13H), 7.49(d, J = 8.1Hz, 1H), 7.55(s, 1H), 7.64(d, J = 8.1Hz, 1H), 7.75(d, J = 7.2Hz, 1H). 13C NMR (75MHz, CDCl3, δ) 65.6, 120.2, 121.4, 121.5, 126.2, 126.9, 127.6, 128.0, 128.1, 128.4, 129.4, 130.7, 139.0, 139.2, 145.2, 151.0, 153.2. GC-MS m/z calcd for C25H17Br: 396.05, found: 396.31(M+) [1].
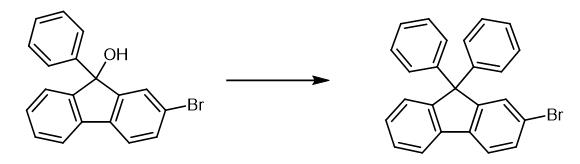
Fig. 2 The synthetic method 2 of 2-Bromo-9,9-diphenylfluorene.
Synthesis of 2-Bromo-9,9-diphenylfluorene 10 g (30 mmol) of 2-bromo-9-phenyl-9H-fluoren-9-ol was dissolved in 60 ml of benzene, and 2.4 ml (45 mmol) of concentrated sulfuric acid diluted with a small amount of benzene was added to the solution. The mixture was stirred for 5 hours at 80° C., and after evaporating the benzene, 1 N sodium hydroxide solution was added to the remaining solution to a pH of around 7. The mixture was extracted 3 times with ethyl acetate (40 ml). The collected organic layer was dried with magnesium sulfate, and the residue obtained by evaporating the solvent was separated and purified by silica gel column chromatography. 6 g of Intermediate N (yield: 50%) [2].
Precautions for the experiment
1. Before the experiment, wear protective glasses, protective clothing, mask, and gloves, and avoid contact with skin.
2. If toxic or irritating substances and harmful substances are encountered during the experiment, the experimental operation should be completed in the glove box when necessary to avoid causing harm to the experimenter.
3. The pipetting nozzle for taking samples should be replaced in time. If necessary, the filter cartridge suction head should be selected as far as possible to avoid cross contamination.
4. When weighing drugs, use weighing paper, take drugs and weigh them in a place without wind to avoid spreading. The container of reagents must be clean and disinfected before use.
5. When taking medicine, try to use multiple medicine spoons separately, clean them after use, dry them, disinfect them and store them.
6. Waste generated after the experiment shall be classified and stored and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
References
[1] Li C S, Tsai Y H, Lee W C, et al. Synthesis and photophysical properties of pyrrole/polycyclic aromatic units hybrid fluorophores[J]. The Journal of organic chemistry, 2010, 75(12): 4004-4013.
[2] Hwang S-H, Kim Y-K, Kwak Y-H, et al. Compound for forming organic film, and organic light emitting device and flat panel display device including the same[P]. U.S. Pat. Appl. Publ., 20090200928, 2009.
You may like
See also
Lastest Price from 2-Bromo-9,9-diphenyl-9H-fluorene manufacturers

US $0.00-0.00/kg2025-03-26
- CAS:
- 474918-32-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons
US $0.00/KG2025-03-21
- CAS:
- 474918-32-6
- Min. Order:
- 1KG
- Purity:
- 98.00%
- Supply Ability:
- 150KG /month