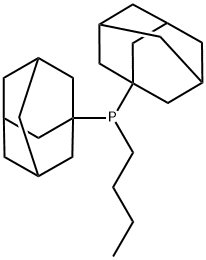
Synthesis and Application of Butyldi-1-adamantylphosphine
Physicochemical properties
Butyldi-1-adamantylphosphine is a white to yellow solid with a melting point of 100°C and an estimated boiling point of 449.6±12.0°C. Store at room temperature, it is air sensitive.
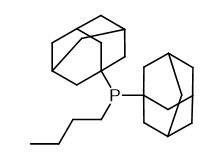
Fig. 1 The structure of Butyldi-1-adamantylphosphine.
Synthetic routes
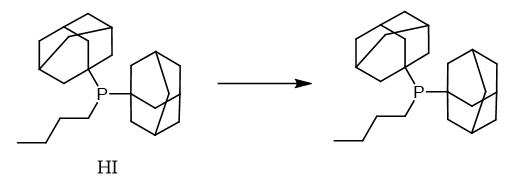
Fig. 2 The synthetic method 1 of Butyldi-1-adamantylphosphine.
Phosphonium salt (2.2 mmol) was added to a cooled solution (- 78 °C) of Et3N (4.44 g, 44 mmol) in di-n-butyl ether (20 mL). The reaction mixture was stirred at -78 °C for 5 h and then allowed to warm gradually to r.t. The solvent was removed under vacuum and the residue was dissolved in degassed EtOH (5 mL). After stirring for 15 min, the solid was filtered off and dried to yield the desired phosphine, which can be further purified by crystallization from EtOH. Di(1-adamantyl)-n-butylphosphine, yield 90%. 31P NMR (C6D6)δ: 24.9. Mp 108-110°C. IR (KBr): 3425 (m, br), 2952 (s), 2847 (s), 2847 (s), 2675 (w), 1446 cm-1 (m). 1H NMR (250 MHz, C6D6): δ = 0.96 (3 H, t, 3JH, H = 7.3 Hz, CH3), 1.35-2.03 (36 H, m, adamantyl-30H, butyl-6H). 13C NMR (62 MHz, C6D6): δ = 41.3 (d, 2JC,P = 11.3 Hz, C-2), 37.4 (C-4), 36.1 (d, 1JC,P = 23.5 Hz, C-1), 33.9 (d, 1JC,P = 26.2 Hz, butyl-α -CH2), 29.1 (d, 3JC,P = 7.6 Hz, C-3), 24.9 (d, 2JC,P = 13.1 Hz, butyl-β -CH2), 17.1 (d, 3JC,P = 21.6 Hz, butyl-γ -CH2), 14.3 (butyl-CH3). MS (EI, 70 eV): m/z (%) = 358 (M+, 60), 135 (Ad+, 100) [1].

Fig. 3 The synthetic method 2 of Butyldi-1-adamantylphosphine.
A 10 mL dried schlenk tube containing a stirring bar was charged with Cu(OTf)2 (13.5 mg, 0.038 mmol) and diphenylphosphine oxide (50.0 mg, 0.25 mmol). Under argon flow dry toluene (2 mL) and TMDS (90 μL, 0.5 mmol) were added, and then the mixture was stirred at 100 ℃ for 9 h. Then, the reaction mixture was cooled to room temperature and N,N-dimethylformamide (degassed, 2 mL), Cs(OH).H2O (43 mg, 0.25 mmol) and 4A molecule cieves (50 mg) were added under Ar flow. After the suspension was stirred for 1 h at room temperature, 1,3-dibromopropane (12 μL, 0.125 mmol) were added under Ar flow. The suspension was heated to 100 oC and stirred for 24 h. Then, the reaction mixture was cooled to 0 ℃ and 3N methanolic KOH (3 mL) was added slowly. After stirring the mixture vigorously for 5 h at room temperature, water (3 mL) was added and the mixture was extracted by ethyl acetate (EA, 2*25 mL). The organic phase was combined and washed by 1N HCl solution (aq., 3 mL) and saturated NaHCO3 solution (aq., 3 mL). Then, the organic phase was dried by Na2SO4 and concentrated under vacuum. The residue was purified bysilica gel column chromatography (EA:pentane = 1:50, rf = 0.6) to give 1,3-bis(diphenylphosphino)propane. Indeed, after in situ generation of di-1-adamantylphosphine or diphenylphosphine, these secondary phosphines are efficiently deprotonated by an excess of base and successfully coupled with four different alkyl halides. Not only mono- but also chelating bisphosphines are obtained with good to excellent yields (51-71%); Yield 71% [2].
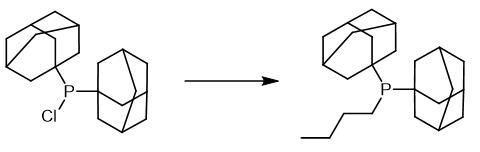
Fig. 4 The synthetic method 3 of Butyldi-1-adamantylphosphine.
To a solution of 1.7 g (5.0 mmol) di-1-adamantyl chlorophosphine in 50 mL of dry THF 5.0 mL of a 1.6 M solution of n-butyllithium in hexane (8.0 mmol) is added at ambient temperature. After 60 min of reflux the solvent is removed under reduced pressure and the residue is purified by distillation. 0.86 g (2.4 mmol) of di-1-adamantyl-n-butylphosphine are obtained. Yield 48%. mp 102 °C [3].
Application
As ligand
Butyldi-1-adamantylphosphine is a novel phosphine ligand, which can be obtained by one-step preparation of bis(1-adamantyl)phosphine and di-n-butyl ether. Although palladium(0)/nickel(0) compounds are known to be the current reaction catalysts, the palladium and nickel catalysts used to activate and refine halogenated aromatic compounds are palladium(II) and/or nickel(II) and palladium(0) and/or nickel(0) complexes. is a novel phosphine ligand, which can be obtained by one-step preparation of bis(1-adamantyl)phosphine and di-n-butyl ether. Although palladium(0)/nickel(0) compounds are known to be the current reaction catalysts, the palladium and nickel catalysts used to activate and refine halogenated aromatic compounds are palladium(II) and/or nickel(II) and palladium(0) and/or nickel(0) complexes. The preparation process of substituted 5,9b-dihydro-4bH-indeno(1,2-b)indol-10-one compound (II) involves taking substituted 2-(2-bromophenyl)-1H-indole (I) and carbon monoxide as starting materials, palladium acetate as catalyst, 1,4-diazabicyclo(2.2.2)octane as base, butyldi-1-adamantylphosphine as ligand and dimethyl sulfoxide or N-methylpyrrolidone as solvent, carrying out reaction, and heating reaction product in reaction vessel at 120 degrees C [4].
As catalyst
Butyldi-1-adamantylphosphine is a catalyst. n-Butylbis(1-adamantyl)phosphine is an electron-rich phosphine ligand used in palladium-catalyzed cross-coupling reactions such as the Heck and Suzuki coupling reactions.
References
[1] Tewari A, Hein M, Zapf A, et al. General synthesis and catalytic applications of di (1-adamantyl) alkylphosphines and their phosphonium salts[J]. Synthesis, 2004, 2004(06): 935-941.
[2] Li Y, Das S, Zhou S, et al. General and selective copper-catalyzed reduction of tertiary and secondary phosphine oxides: Convenient synthesis of phosphines[J]. Journal of the American Chemical Society, 2012, 134(23): 9727-9732.
[3] Ehrentraut A, Zapf A, Beller M. Palladium‐Catalyzed Reactions for Fine Chemical Synthesis. Part 18. A New Efficient Palladium Catalyst for Heck Reactions of Deactivated Aryl Chlorides[J]. ChemInform, 2001, 32(8).
[4] Guo S, Wang F, Tao L, Fan X. Preparing substituted 5,9b-dihydro-4bH-indeno(1,2-b)indol-10-one compound by taking substituted 2-(2-bromophenyl)-1H-indole and carbon monoxide as starting materials, palladium acetate as catalyst and dimethyl sulfoxide as solvent[P]. Faming Zhuanli Shenqing, 106349150, 2017.
See also
Lastest Price from Butyldi-1-adamantylphosphine manufacturers

US $0.00-0.00/KG2025-03-14
- CAS:
- 321921-71-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt
US $0.00/kg2025-03-03
- CAS:
- 321921-71-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS