Synthesis and Detection of 1,3-Dimethylpentanamine Hydrochloride
Generally speaking
1,3-Dimethylpentylamine is a pseudo-adrenergic drug introduced by Lilly Company in the United States in the mid-1940s under the trade name Forthane. 1,3-dimethylpentylamine and its hydrochloride have obvious curative effects in the treatment of nasal congestion and gingival hyperplasia, and are much less stimulating to the mind than pseudoephedrine and amphetamine, and their unique pharmacological advantages cause attached great importance to the experts.

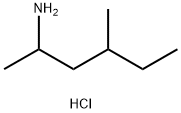
Fig. 1 The structure of 1,3-Dimethylpentanamine Hydrochloride.
Synthetic routes
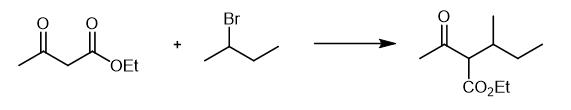
Fig. 2 The synthetic step 1 of 1,3-Dimethylpentanamine Hydrochloride.
At room temperature, 800 mL absolute ethanol was added into a 2 L three neck flask, stirred, and 35.38 g (1.538 mol) of metal sodium was added in batches to obtain a colorless transparent liquid. Cool slightly, add 200g (1.537 rnol) of ethyl acetoacetate, continue heating slowly, and add 232g (1.693 mol) of 2-bromobutane drop under weak reflux. Continue heating and react for 8 h at 80~84 ℃. Stop heating, cool naturally to room temperature, leave it still, filter by suction, and concentrate the yellow green filtrate under reduced pressure to obtain 241.4 g of light yellow viscous solid in 84.3% yield [1].
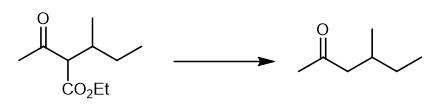
Fig. 3 The synthetic step 2 of 1,3-Dimethylpentanamine Hydrochloride.
At room temperature, 1304 mL of 5% sodium hydroxide solution and 241.4 g of 3-isobutyl ethyl acetoacetate (4) were added into a 2L three necked flask, and stirred for 4 h. Drip 50% (wt) sulfuric acid solution, adjust the pH to 4.0, gradually increase the temperature, gradually increase the pH, react for 4 hours, distill and collect 84~86 ℃ distillate, extract the collected liquid with saturated calcium chloride solution, collect 140 g of organic phase, add 15 g of anhydrous CaCl2 to dry, filter, and distill with a prick distiller, collect 130~140 ℃ distillate (boiling point 130~140 ℃ in literature), and obtain 67.2 g of product with a yield of 45.4% [1].

Fig. 4 The synthetic step 3 of 1,3-Dimethylpentanamine Hydrochloride.
At room temperature, 38.6 g (0.34 mol) of 4-methyl-2-hexanone (3), 60.18 g (0.36 moI) of hydroxylamine sulfate, and 115 mL of water are added into a 250 mL three neck flask. Under stirring, about 40 rnL of saturated sodium hydroxide solution is added dropwise, and the pH of the reaction solution is adjusted to 7.0. Continue the reaction for 4 h, extract, and use water for organic phase (10 mL × 3) Wash, dry with anhydrous magnesium sulfate, and filter out the desiccant to obtain 70.1g of light yellow transparent liquid with a yield of 80.3%. 1H—NMR(CDCl3, 300 MHz) δ 0.84-0.93(m, 6H), 1.13-1.24(m, 1H), 1.26-1.41(m, 1H), 1.63-1.79(m, 1H), 1.87(s, 3H), 1.94-2.01(m, 1H), 2.15-2.24(m, 1H), 2.28-2.34(m, 1H). ESI-MS(m/z): 130(M+1) [1].
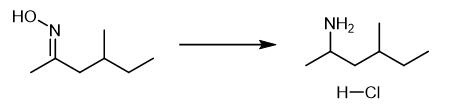
Fig. 5 The synthetic step 4 of 1,3-Dimethylpentanamine Hydrochloride.
At room temperature, add 30 g (0.23 mol) of 4-methyl-2-monoketoxime (4) and 670 mL of absolute ethanol into a 2L three neck flask. Heat slowly. Under weak reflux, slowly add 75 g (3.26 mol) of metal sodium. Heat is released obviously. Stop heating. Add metal sodium in 1h. Heat to reflux state and react for 1.5 h, stop heating, add water, stir to obtain light yellow transparent liquid, and collect distillate below 120 ℃ under normal pressure; Adjust pH to 4.0 with 6 mol L-1 hydrochloric acid solution, and concentrate under reduced pressure to obtain crude light yellow solid. Recrystallize with ethanol to obtain 12.2 g of white solid with yield of 40.0% and purity of 99.3%. MP: 128~132 ℃ (literature mp 130~135 ℃). 1H-NMR(CDCl3, 300 MHz) δ 0.86-0.94(m, 6H), 1.12-1.28(m, 1H), 1.31-1.53(m, 5H), 1.57-1.71(m, 1.5H), 1.76-1.88(m, 0.5H), 3.40(m, 1H), 8. 33(s, 3H). 13C-NMR(CDCl3, 75 MHz) δ 10.96, 18.48, 19.30, 29.43, 30.76, 41.93, 46.60. ESI-MS(m/z):116(M+ 1) [1].
Detection
In an earlier study, we developed two sensitive and reliable procedures for gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of methylhexaneamine (MHA) in P. graveolens plant materials and volatile oils. None of the analyzed plant materials or oils showed any detectable levels of MHA which was further substantiated by high resolution liquid chromatography-quantum time of flight-mass spectrometry (LC-QTOF-MS) analysis with a limit of detection of 10ppb. However, other laboratories (two studies) reported the presence of MHA in some samples of P. graveolens and pelargonium oil acquired by the investigators from China. Because of the controversy of whether Pelargonium species or pelargonium oil contains MHA, it was recommended that splits of multiple samples be analyzed by different laboratories. In this investigation, multiple plant materials and oil samples were collected from around the world. These samples were submitted to four different sites for analysis. All sites adopted a similar extraction method. All the analysis sites used LC-MS/MS or LC-QTOF-MS and detection limit was set close to the 10ng/mL as previously reported. A total of 18 plant samples belonging to 6 different Pelargonium species and 9 oils from different locations around the world were split among 4 different analytical laboratories for analysis (each lab received the same samples). None of the laboratories detected MHA in any of the samples at or around the 10ppb detection level of the procedure used [2].
References
[1] Lu Q, Wang Y. Synthesis technology of 1,3-dimethylpentylamine hydrochloride for drug [J]. Zhongnan Yaoxue, 2011, 9(11), 814-817.
[2] ElSohly M A, Gul W, Tolbert C, et al. Methylhexanamine is not detectable in Pelargonium or Geranium species and their essential oils: A multi‐centre investigation[J]. Drug testing and analysis, 2015, 7(7): 645-654.
Related articles And Qustion
See also
Lastest Price from 4-Methyl-2-hexanamine hydrochloride manufacturers

US $10.00/ASSAYS2025-08-21
- CAS:
- 13803-74-2
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $0.00/kg2025-04-24
- CAS:
- 13803-74-2
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg