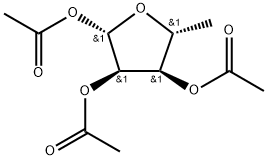
Synthesis of Capecitabine with 1,2,3-Triacetyl-5-deoxy-D-ribose
1,2,3-Triacetyl-5-deoxy-D-ribose (5-deoxy-1,2,3-triacetyl-D-ribose) is intermediates for the preparation of such antineoplastic agents like capecitabine, which is a prodrug of 5-fluorouracil, for the treatment of paclitaxel and doxorubicin drugs ineffective advanced primary or metastatic breast cancer [1].
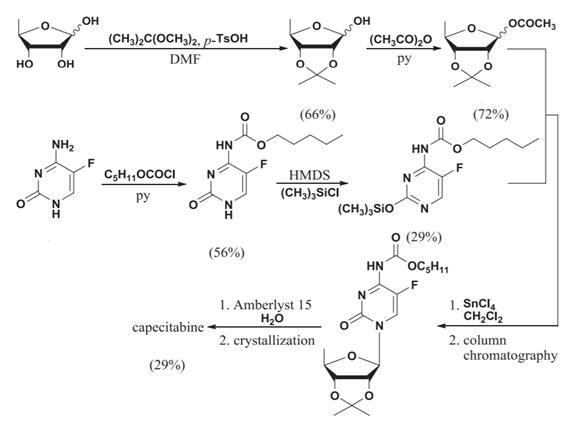
Scheme 1 The synthesis route of capecitabine from 1,2,3-Triacetyl-5-deoxy-D-ribose
Patrizia Ferraboschi and his colleagues [2] summarized lots of synthesis route for synthesis of antitumor pyrimidine nucleosides, including the synthesis route of Capecitabine (Scheme 1) from 1,2,3-Triacetyl-5-deoxy-D-ribose.
The synthesis is designed starting from protected 5-deoxy-Dribose, which react with 2,2-dimethoxypropane in the presence DMF solvent to afford the compound; then 2,3-O-isopropylidene-5-deoxy-Dribose, acetic anhydride and pyridine react in the presence of a suitable organic solvent to afford the compound 2,3-O-isopropylidene-3-O-acetyl-5-deoxy-D-ribose.
In other side, 5-fluorocytidine was charged into a flask followed by charging of pyridine(py), and n-pentyl chloroformate was added to the reaction mixture at 25℃ ~ to 30℃ over 10 minutes. After completion of the addition, the reaction solution was heated to 100~110℃ followed by stirring for 2 hours to get N-f(pentyloxy) carbonyl]-5- fluorocytosine, which was charged into a flask followed by charging of hexamethyldisilazane (HMDS) and trimethylsilylchloride (TMS-CI), and the reaction mixture was heated to 80℃ and stirred for 2 hours. The resultant reaction solution was cooled to 50℃ and purified to afford silylated N-[(pentyloxy) carbonyl]-5-fluorocytosine.
Then, dichloromethane and 2,3-O-isopropylidene-3-O-acetyl-5-deoxy-Dribose of were charged to the above obtained silylated N-[(pentyloxy) carbonyl]-5-fluorocytosine compound. The reaction mixture was stirred at 25-35℃ for 10 minutes and then cooled to 0-5℃. Stannic chloride (SnCl4) was added over 10 minutes to the above reaction mixture at 0-5°C and stirred for 2 hours. The temperature of the reaction mixture was raised to 25-30℃ and stirred for 1 hour. After completion of the reaction, the reaction was decomposed by the charging of sodium bicarbonate and stirred at 25-30℃ for 1 hour. The product was gained after a series complicated purification processes to prepare Capecitabine by adding ethanol, Amberlyst™ 15 catalyst and demineralized water in and kept stirring for 9 hours at 25-30℃ [3].
5-deoxy-1,2,3-triacetyl-D-ribose is the important intermediate of Capecitabine synthesis, but the following each step is still key to get the final Capecitabine.
Reference
[1] Guangyan Wu, 1,2,3-Triacetyl-5-deoxy-D-ribose preparation method, CN103772450A.
[2] Patrizia Ferraboschi, Samuele Ciceri, and Paride Grisenti, Synthesis of Antitumor Fluorinated Pyrimidine Nucleosides, Organic Preparations and Procedures International, 49:1–85, 2017,
[3] Raghavendracharyulu Venkata Palle etc., Process for preparing capecitabine, WO 2008131062A2.
Related articles And Qustion
Lastest Price from 1,2,3-Triacetyl-5-deoxy-D-ribose manufacturers

US $0.00/kg2025-09-01
- CAS:
- 62211-93-2
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $5.00-0.50/KG2025-05-16
- CAS:
- 62211-93-2
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg