Uses of 4-Bromo-2-methylbenzoic acid as a Synthetic Intermediate
4-Bromo-2-methylbenzoic acid(4-Bromo-2-methylbenz;4-Bromo-o-toluicacid; 5-Bromo-2- carboxytoluene; 4-Brom-2-methylbenzoic acid; C8H7BrO2)[1] is a commom synthetic intermediate or materials in organic synthesis.
4-Bromo-2-methylbenzoic acid have applied to synthesize phenoxybenzoylphenyl acetic acids by Friedel-Crafts acylation with diphenyl ether and a cross-coupling reaction with ethynyltrimethylsilane [2]. In these process, the 1- and 4- were active sites and participate acylation and nucleophile substitution reaction, respectively.
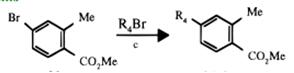
Starting with 4-bromo-2-methylbenzoic acid methyl ester from the esterification reaction of 4-bromo-2- methylbenzoic acid, 4-alkyl-2-methylbenzoic acid methyl esters could be produced by the cross-coupling reaction[3].
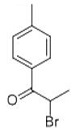
Isoindolinone derivatives could be prepared initiated from 4-bromo-2-methylbenzoic acid. The 1- of starting material have been esterified, which was further brominated. The intermediate was then subjected to a reflux with isopropyl amine to generate the isoindolinone fragment, which could further participate coupling reactions by 4-bromo [4].

4-Bromo-2-methylbenzoic acidwas regioselectively chlorinated by N-chlorosuccinimide (NCS), which further reacted with n-propylamine to obtain benzamide. Direct benzylic lithiation of benzamide with LDA in the presence of DMF would afford 6-bromo-8-chloro-N-propylisoquinolone, which could derived a series of N-propyl-8-chloro- 6-substituted isoquinolones[5].
By three step methodology, 4-bromo-2-methylbenzoic acid could be transformed to 2,5-bis(4-bromo-2-methylphenyl)-1,3,4-oxadiazole or 2,5-bis(4-bromo-2-methylphenyl)- 1,3,4-thiadiazole based on one of the most popular catalytic methods of C–C bond construction – the Suzuki cross-coupling reaction[6].
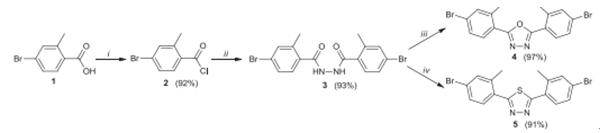
Moreover, using 4-bromo-2-methylbenzoic acid as material, mesogen-jacketed liquid crystalline polymer,such as poly[4,4′-bis(4-butoxyphenyloxycarbonyl)-2-vinylbiphenyl] and poly[4,4′-bis (4-butoxyphenyloxycarbonyl)-3- vinylbiphenyl] could be produced through multistep coupling reaction[7].
In conclusion, 4-bromo-2-methylbenzoic acid with three active sites can generate multiple coupling or acylation reaction to produce various aim product. Thus, 4-bromo-2-methylbenzoic acid is a important intermediate in organic synthesis.
References
[1] http://www.basechem.org/chemical/31663.
[2] Salem, O. I., Frotscher, M., Scherer, C., Neugebauer, A., Biemel, K., Streiber, M., Hartmann, R. W. (2006). Novel 5α-reductase inhibitors: synthesis, structure− activity studies, and pharmacokinetic profile of phenoxybenzoylphenyl acetic acids. Journal of medicinal chemistry, 49(2), 748-759.
[3] Baumgarth, M., Beier, N., Gericke, R. (1997). (2-Methyl-5-(methylsulfonyl) benzoyl) guanidine Na+/H+ antiporter inhibitors. Journal of medicinal chemistry, 40(13), 2017-2034.
[4] hi Cho, G., Kim, T., Son, W. S., Seo, S. H., Min, S. J., Cho, Y. S., Pae, A. N. (2015). Synthesis and biological evaluation of aryl isoxazole derivatives as metabotropic glutamate receptor 1 antagonists: A potential treatment for neuropathic pain. Bioorganic & medicinal chemistry letters, 25(6), 1324-1328
See also
Lastest Price from 3-oxo-2-phenylbutanoic acid manufacturers

US $0.00/kg2025-03-18
- CAS:
- 4433-88-9
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10T

US $1.00/KG2024-05-29
- CAS:
- 4433-88-9
- Min. Order:
- 100g/ml
- Purity:
- 99.9%
- Supply Ability:
- 50000 tons